Article by Lalita Panicker, Consulting Editor, Views and Editor, Insight, Hindustan Times, New Delhi
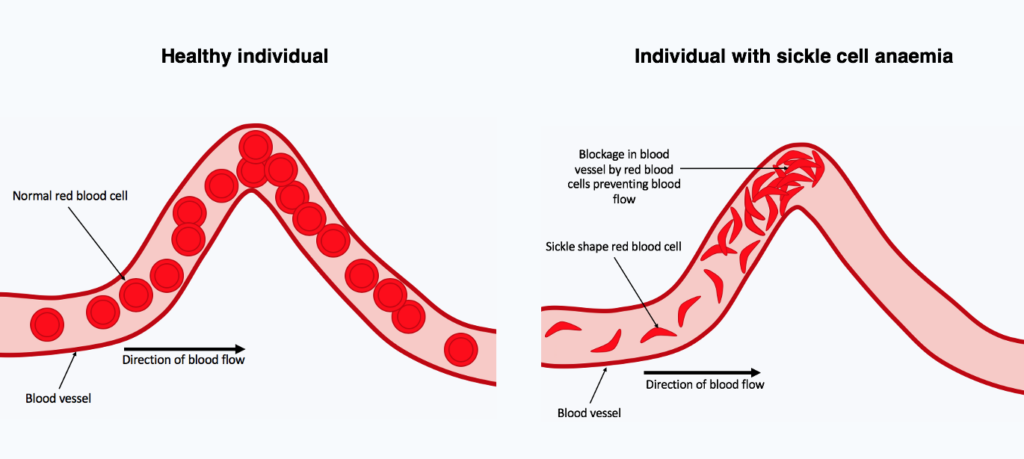
Last year, the U.S. Food and Drug Administration approved two gene therapy procedures that can treat and, in some cases, essentially cure sickle cell disease. This genetic blood disorder causes pain and anaemia in millions and still kills nearly 375,000 people worldwide every year. But the groundbreaking treatments require risky chemotherapy and cost some $2 million per person, putting them out of reach of the vast majority of sickle cell patients. Now, pharmaceutical researchers are reporting a potential oral drug that restores healthy blood cells in animal models of the disease.
Unlike a one-time gene therapy whose benefits could last decades, the new compound might have to be taken periodically for life and it hasn’t even begun safety testing in humans. But the experimental drug offers hope that sickle cell disease could one day be widely and cheaply treatable with a simple pill.
The new compound helps turn on the production of a form of haemoglobin that is used by foetuses but is normally suppressed shortly after birth. The team found that when the compound was given to immature red blood cells, it raised foetal haemoglobin production from a baseline of 17% to 45%. According to the researchers, the latter is at a level that would produce functional red blood cells if the approach translated into humans.
////
In 19th century New York City, Theodore Gaillard Thomas enjoyed an unusual level of fame for a gynaecologist. The reason, oddly enough, was milk. Between 1873 and 1880, the daring idea of transfusing milk into the body as a substitute for blood was being tested across the U.S. Thomas was the most outspoken advocate of the practice. www.science.org/content/article/ultimate-blood-substitute-us-military-betting-46-million?
At the time, severe bleeding was often a death sentence. Blood transfusion was practiced, but it was something of a crapshoot. Medical science was still 3 decades removed from discovering blood types. Patients who received mismatched blood suffered discoloured urine, itching, and a sometimes-fatal complication: haemolytic shock, wherein their own immune systems attacked the transfused cells.
Doctors in the U.S. were looking for something less risky to stabilize a haemorrhaging patient. Thomas was sure milk was the answer. In 1875, he injected 175 millilitres of cow’s milk into a woman suffering from severe uterine bleeding after an operation to remove her cancerous ovaries. At first, he wrote, the patient “complained that her head felt like bursting.” She soon developed a high fever and an abnormally high heart rate, but recovered a week later. Thomas subsequently performed seven separate milk transfusions, publishing his results in several medical journals, and predicted their “brilliant and useful future.”
It was not to be: Saline solutions, still used today, were introduced the next decade as a much less dangerous, if imperfect, stopgap measure for emergency bleeding.
The need for blood substitutes, however, survives. And last year in a downtown Baltimore laboratory, a white rabbit embodied the latest hope.
The bunny huddled in a black metal cage, a catheter going straight into its carotid artery. Days before, a portion of its blood had been siphoned out and replaced with an experimental blood substitute called ErythroMer. It is decidedly not milk. Developed by Allan Doctor, a bespectacled 61-year-old physician-researcher at the University of Maryland (UMD) School of Medicine, and colleagues, ErythroMer is made from “recycled” human haemoglobin—the protein in red blood cells that carries oxygen from the lungs to the rest of the body—wrapped in a membrane to mimic a tiny cell. In the rabbit, the transfusion appeared to be working. The animal’s heart rate and blood pressure, displayed on a small monitor nearby, looked just fine.
Doctor is as fervent an advocate for haemoglobinase oxygen carriers (HBOCs), as ErythroMer and its predecessors are more formally known, as Thomas was for lacteal transfusions. Donated blood has a shelf life of just 42 days. There’s also not enough, even in developed countries with well-organized blood donation systems: In January 2022, the American Red Cross, which distributes 40% of the country’s donor blood, declared the first-ever national blood crisis, as its supply—especially precious O-negative blood, the universal type—dipped dangerously low. Meanwhile, haemorrhagic shock caused by severe blood loss kills some 20,000 people in the U.S., and 2 million globally, every year.
Artificial “blood” could, perhaps, fill the void. In settings where fresh blood is hard to come by, such as battlefields and rural areas (where ambulance wait times are sometimes as high as 45 minutes), ErythroMer could be given on the fly to maintain the vital flow of oxygen to organs until someone reaches a hospital. It’s a freeze-dried powder that remains usable for years and can be reconstituted by simply mixing it with widely available saline. And ErythroMer should be safe for any blood type, because its membrane doesn’t include the red blood cell surface proteins that cause mismatches.
For now, no human blood substitute is commercially available in the U.S. “There’s a real gap here where we don’t have access to blood for people bleeding to death outside of the hospital,” says Doctor, who co-founded and is chief science officer of KaloCyte, a company hoping to develop ErythroMer into a commercial product.
Last year, the Defense Advanced Research Projects Agency (DARPA) announced a $46 million grant to a UMD-led consortium to develop a shelf stable, field-deployable whole blood substitute with ErythroMer as its core. “ErythroMer … is notable for its detailed emulation of natural red blood cell function,” says Jean-Paul Chretien, program manager in DARPA’s Biological Technologies Office and a former Navy medical officer.
So far Doctor’s creation remains in animal testing, but it isn’t the only effort to package haemoglobin inside lipids to fashion a viable blood substitute. A rival product in Japan has already been tested in a few people and generally appears safe.
But the success of these new products is far from guaranteed. Barely 2 decades ago, earlier formulations of HBOCs were scuttled or sidelined after trial participants died. Subsequent attempts haven’t fared much better. The most advanced HBOC to date, approved for people in South Africa and Russia, has struggled amid concerns about side effects.
Erythromer doesn’t have clinical success stories yet, but it now has DARPA’s millions. Doctor believes it can avoid the toxicity of pure hemoglobin products—by even more closely imitating nature and enclosing the molecules like red blood cells do.
ErythroMer is still in the early stages of testing. The latest preclinical data demonstrated effective oxygen delivery in mice that had 70% of their blood volume replaced with ErythroMer. And in rabbits, when half of their blood volume is removed, infusing fluid containing ErythroMer resuscitated the animals, as real blood does. Doctor hopes to perform an initial safety test of ErythroMer in healthy humans, perhaps before the DARPA grant ends in 4 years.
KaloCyte’s product isn’t the only encapsulated haemoglobin around. In Japan, a team led by chemist Hiromi Sakai at Nara Medical University has done something similar by enclosing haemoglobin in lipid vesicles. These HbVs, or haemoglobin vesicles, are simpler than ErythroMer, without its membrane additions, but the product is further along in development.
A phase 1 safety trial in men was cut short by the pandemic coronavirus in 2020, but initial results appeared encouraging. Side effects included rash and fever, but they were temporary, and there were no clinically significant changes in participants’ vital signs. “This finding suggests that, compared with the first generation of HBOCs, HbVs have less potential, if any, for myocardial infarction,” wrote Sakai and his colleagues in a 2022 research letter.
Other innovative idea to replace to replace red blood cells are also still being explored. A company called Hemarina is developing a product that makes use of a free haemoglobin found in the blood of a marine worm species.
What are the odds any of these new HBOCs cracks the blood substitute code? At least one expert scarred by the field’s failures is sceptical. “A sheathed haemoglobin will probably fill too much space [in a blood vessel] and not carry enough oxygen to be useful,” says John Hess, a physician and University of Washington professor of laboratory medicine. A retired U.S. Army colonel, Hess ran the army’s blood product development program from 1991 to 2001. He halted the military’s work on haemoglobin products in 1997. “I’m not saying there cannot be a safe HBOC,” he adds. “I certainly could not see a pathway to one, and shut down the army program on that basis.”
Others see more promise. “We are close enough in knowledge with how these products work that with the appropriate financing, one or more will be on the market,” Vandegriff says. “It remains a highly unmet medical need.”
After all, as physicians have developed new and better ways to help people threatened by severe bleeding, the demand for an emergency blood substitute has only got stronger. “There isn’t actually enough O negative blood to just give everybody,” Doctor says. “You need blood that’s shelf stable and universal donor.”
He hopes ErythroMer will prove the doubters like Hess wrong. For now, Doctor monitors the health of his rabbits.


