Article by Lalita Panicker, Consulting Editor, Views and Editor, Insight, Hindustan Times, New Delhi
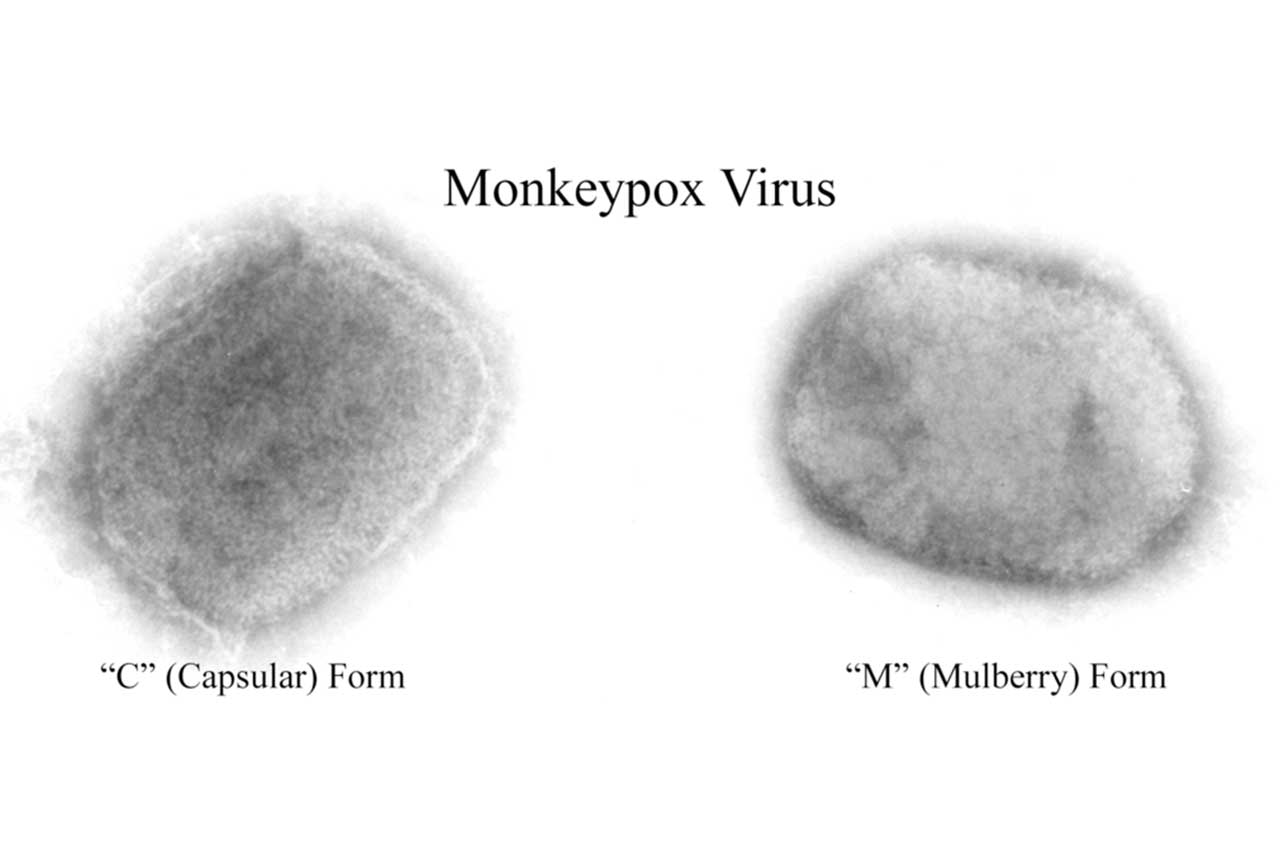
Researchers and public health officials in Africa are intensifying their battle against mpox, a neglected infectious disease that has circulated for a long time on the continent and suddenly gained notoriety in 2022 when it started to spread rapidly in Europe and North America. https://www.science.org/content/article/africa-intensifies-battle-against-mpox-alarming-outbreaks-continue?
At a meeting last week in Kinshasa, the capital of the DRC, scientists from 10 affected African countries reviewed an alarming rise of cases on the continent, discussed plans to improve mpox surveillance, introduce vaccination, and launched an African-led research consortium.
The meeting, convened by the Africa Centres for Disease Control and Prevention (Africa CDC) and the first of its kind on the continent, came as more evidence pours in that in Africa, too, mpox is sexually transmitted—and not just among men who have sex with men (MSM), the community most affected during the recent global outbreak.
Researchers in Africa have documented sexual transmission as well, and a preprint posted on 14 April on medRxiv by the new Mpox Research Consortium warned that an outbreak of a new strain of the virus, in Kamituga, a mining region in the eastern DRC, appears to be driven in part by men hiring women sex workers. That strain has the potential to trigger another worldwide outbreak if it is not contained, the researchers caution. “The sexual transmission is making it all the more alarming,” said Africa CDC head Jean Kaseya. “We must have a coordinated response.”
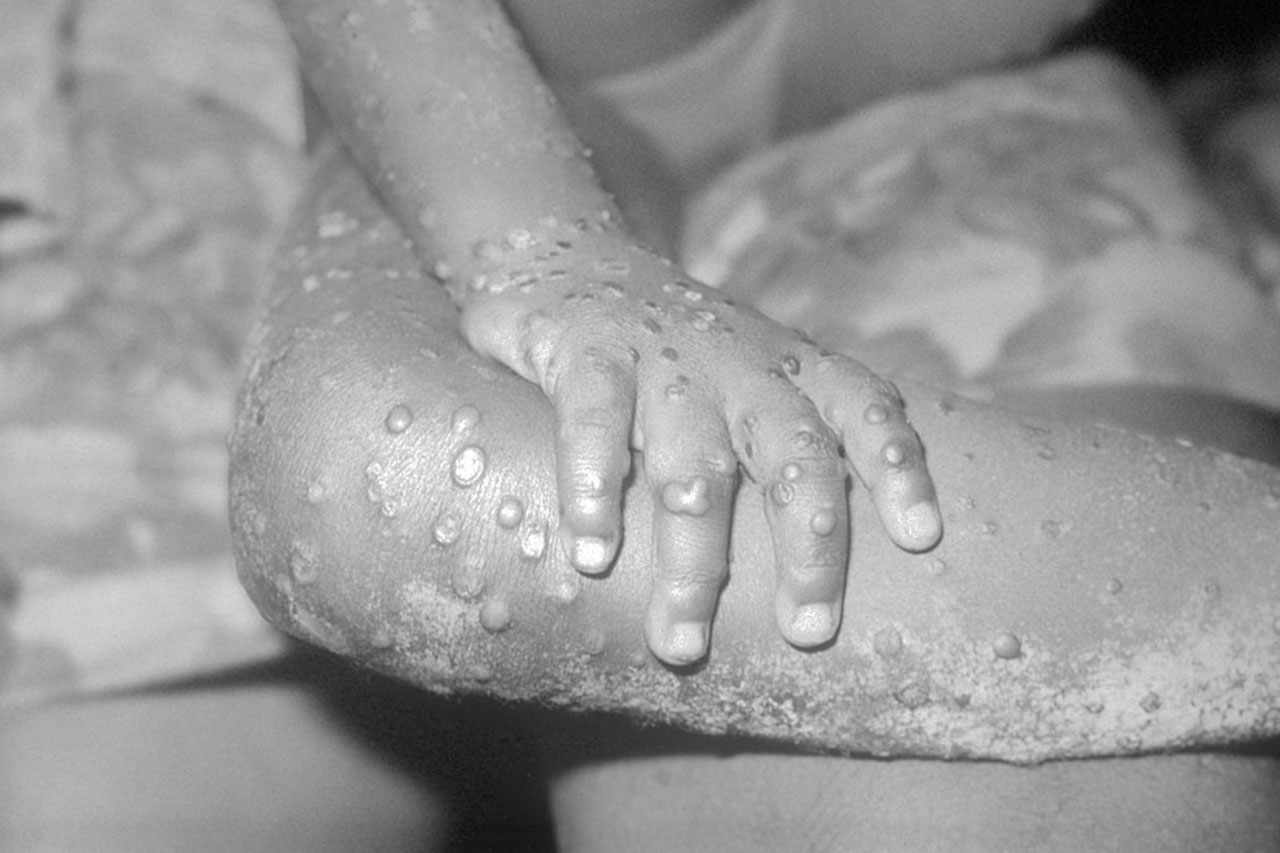
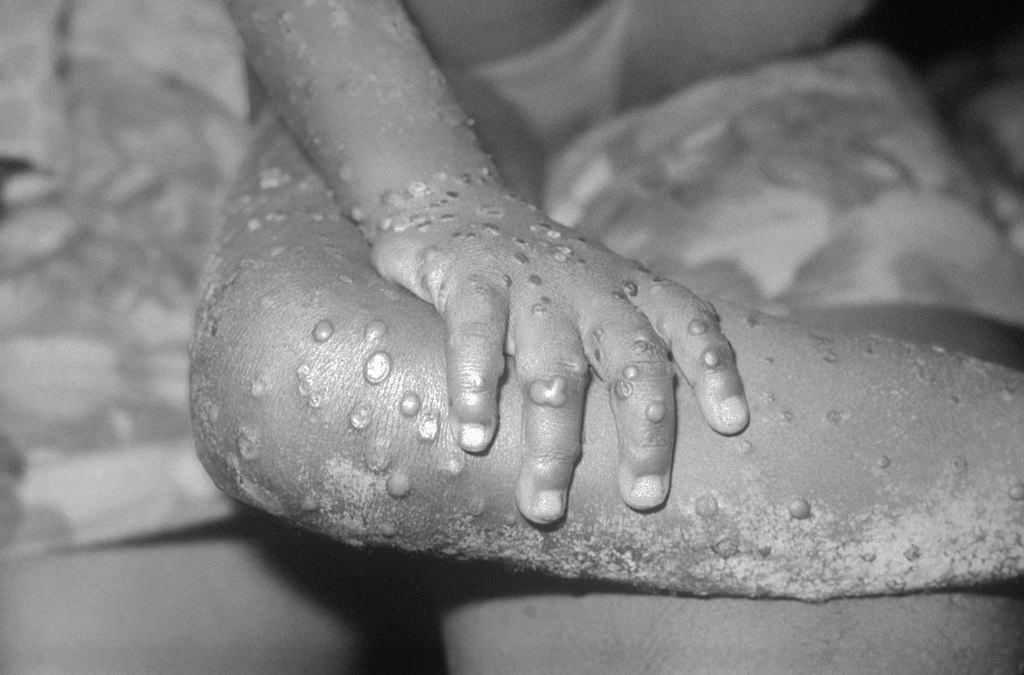
But the battle to contain mpox in Africa has many challenges not seen elsewhere. It continues to see spill over from animal reservoirs there to humans, although which host species are most important is unclear. Many cases go unrecognized because the disease resembles chickenpox, often occurs in remote villages, and is not easily diagnosed. Vaccines have yet to be used in Africa, and stigma about the disease within two populations at high risk, MSM and sex workers, remains high in most countries.
The global outbreak, first recognized in May 2022, affected 117 countries, causing often painful lesions in nearly 100,000 people and more than 600 deaths. After prevention campaigns and vaccines were rolled out, however, cases started to drop rapidly. The Public Health Emergency of International Concern, declared by the World Health Organization (WHO) in July 2022, was ended 10 months later. In February, WHO reported fewer than 500 confirmed cases outside of Africa.
But the DRC alone—where the first human case was reported in 1970—had 14,434 suspected cases and 728 deaths in 2023. The pace of infections in the country appears to be picking up: There have been nearly 5000 so far this year, three times the number seen in the same period in 2023. Cameroon, the Central African Republic, Nigeria, the Republic of the Congo, Ghana, and Liberia together reported another 400 cases last year. All these numbers are likely vast underestimates because surveillance is spotty.
Sequencing of the virus from 22 people in Kamituga (DRC) showed it likely spilled over from an animal host a month before the first case was noticed and then acquired mutations tied to human-to-human transmission. Because Kamituga is a hub for migrants, the outbreak “harbours the potential to spread nationally and internationally,” the authors warned.
To control outbreaks, African countries hope to soon start offering people an mpox vaccine that went into wide-scale use in wealthy countries for the first time during the global outbreak. Made by Bavarian Nordic, it contains a weakened version of the vaccinia virus used in the smallpox vaccine. A second weakened vaccinia-based vaccine, known as LC16m8, is made by the Chemo-Sero-Therapeutic Research Institute in Japan. WHO’s Strategic Advisory Group of Experts on Immunization in March made a global recommendation for the use of the weakened vaccines to help contain mpox outbreaks and as preventive shots for those at high-risk of becoming infected.
Regulatory authorities in Nigeria and the DRC have recently approved both weakened virus vaccines and the U.S. government has donated 10,000 doses of the Bavarian Nordic product to Nigeria and 50,000 to the DRC, says Rosamund Lewis, who oversees mpox for WHO.
////
A team in China probing the guts of local mosquitoes has found a potential helper in the fight against two human diseases. Researchers identified a new bacterium that disables the viruses responsible for dengue and Zika before they can establish an infection in the insects. Although early stage, the work, reported this week in Science, paves the way for studying the bacterium’s effect on disease transmission in the real world. www.science.org/content/article/bacteria-found-mosquito-guts-could-help-scientists-fight-dengue-zika?
It wouldn’t be the first time a microbe is used to thwart mosquito-borne diseases. About 15 years ago, researchers discovered that a different bacterium, Wolbachia, reduces the insects’ ability to transmit dengue, among other viruses. Following successful field trials, Wolbachia is now used to help control dengue in more than a dozen countries. But an extra weapon to help control mosquito-borne diseases is welcome—especially as the insects become resistant to current insecticides.
The Chinese team launched a search for microbes that, like Wolbachia, might help control the disease from within the mosquito. They focused on Yunnan province, parts of which are infested with Asian tiger mosquitoes (Aedes albopictus), a species that can transmit dengue and Zika viruses.
Using themselves as bait, team members waited for mosquitoes to approach before hoovering them up with handheld aspirators, says study co-author Gong Cheng of Tsinghua University. Back at the lab, they identified 55 bacterial species in these insects’ guts. To see whether any had antiviral properties, the researchers treated A. albopictus with antibiotics to decimate their existing gut microbes. Then, they fed them individual bacterial species plus a meal of human blood laced with dengue virus. A control group received antibiotics and virus but no bacteria.
One species in the genus Rosenbergiella, which is often found in flower nectar, caught their attention. Mosquitoes given that bacterium, dubbed Rosenbergiella_YN46, were significantly less likely than controls to have detectable virus in them 1 week later. The researchers replicated the effect in the yellow fever mosquito, A. aegypti, which is dengue’s other main spreader. Rosenbergiella_YN46 also hampered Zika virus in both species, and it thwarted infection in A. albopictus mosquitoes that bit dengue-infected mice. The researchers traced the effect to an enzyme secreted by the bacterium that makes the gut acidic, apparently deforming proteins that viruses need to invade insect cells.
Finally, in outdoor mesh tents, Cheng’s team let wild-caught A. albopictus mosquitoes lay eggs in pools of water spiked with Rosenbergiella_YN46. In this more realistic scenario, larvae and adults ended up with the bacterium in their guts—and were less susceptible to dengue infection than were mosquitoes raised with Rosenbergiella-free water.
Before releasing the bacterium more widely, researchers need to establish whether it affects other insects, and how it interacts with other microbes, including Wolbachia—which, unlike Rosenbergiella, resides inside cells—notes Steven Sinkins, a vector biologist at the University of Glasgow.
Getting the bacterium into enough wild mosquitoes to block disease transmission will likely be harder than it is for Wolbachia, which spreads rapidly thanks to some unusual effects on mosquito reproduction. Cheng’s team plans to test Rosenbergiella_YN46–laced ovitraps—containers of water where mosquitoes lay eggs—in villages in Yunnan, and to track dengue incidence in local communities.
Cheng also wants to identify plants that contain the bacterium. If it turns out wild mosquitoes pick up Rosenbergiella YN46 via nectar, he speculates, perhaps “we may cultivate the plants” in dengue-rife regions to try to reduce transmission that way.
////
In March 2022, when the pandemic was still raging, the messenger RNA (mRNA) company Moderna announced it would build a $500 million plant in Kenya to manufacture half a billion doses of its COVID-19 vaccine annually.
But Moderna may never break ground on the Kenya factory. On 11 April, the company said it had “paused its efforts” because not a single African country had ordered its COVID-19 vaccine since 2022, leading to $1 billion in losses and write-offs. The move triggered a bitter reaction from the Africa Centres for Disease Control and Prevention (Africa CDC), which said, “Moderna is abandoning a commitment to build highly needed and relevant vaccine manufacturing capabilities in Africa.” www.science.org/content/article/plans-expand-african-vaccine-production-face-steep-hurdles?
Producing more vaccines in Africa “is a moral imperative, and it’s economically difficult,” says Martin Friede, head of vaccine research at the World Health Organization (WHO). “So we are struck between a rock and a hard place.”
The COVID-19 pandemic exposed the harsh inequities in vaccine access like never before. Rich countries purchased far more doses than they needed, whereas India, home of the largest producers of shots meant for developing countries, blocked their export in March 2021 to deal with its own COVID-19 surge. By the end of 2021, when wealthy countries had fully vaccinated most of their populations, fewer than 20% of Africans had received at least one dose.
One early effort was an mRNA “techno-logy transfer hub” in South Africa, led by Friede. Over the past 3 years, the hub has trained scientists from 15 countries, including six in Africa, to produce mRNA vaccines themselves. Today, “Each of these countries is facing the situation of, ‘OK, we requested mRNA technology, thank you, we appreciate it. Now, what do we do with it?’” Friede says. “If you are not producing a product that is being procured on a day-to-day basis, after a couple of years, you will go bankrupt.” Friede says he understands Moderna’s decision to halt its Kenya plans.
Several other programs have launched. In 2021, the African Union and Africa CDC launched the Partnership for African Vaccine Manufacturing (PAVM), whose goal is to have 60% of the continent’s vaccine doses produced there by 2040. That bold ambition has “galvanized the continent,” says John Nkengasong, a Cameroon-born virologist who led Africa CDC at the time and now heads the U.S. President’s Emergency Plan for AIDS Relief.
PAVM will coordinate fundraising for the effort and wants to see seven vaccine technologies pursued, including mRNA, traditional inactivated and weakened viruses, and harmless viral vectors that carry genes from dangerous pathogens. Some 30 projects, including the three BioNTech facilities, are underway, says virologist Nicaise Ndembi, who heads PAVM.
Another major initiative came in December 2023 from Gavi, which currently purchases about half the vaccine doses used in Africa. Gavi’s $1 billion commitment is for the African Vaccine Manufacturing Accelerator, a plan to speed production of shots to protect against 11 diseases. The money will be doled out to local companies in a complicated scheme that rewards them if their products pass muster with WHO’s strict quality standards and awards extra bonuses for those who sell to Gavi or the world’s other major vaccine purchaser, UNICEF. The funding aims to help at least four African vaccine makers together produce 800 million doses annually within a decade.
Yet these plans face significant obstacles, according to a recent analysis by Africa CDC, CHAI, and PATH, another U.S. non-profit. Few African companies have the capacity to produce antigens, the pathogen components that trigger immune responses and are the core ingredient of any vaccine, the groups said. For that, the firms need foreign companies to transfer technologies, but few such deals exist, and securing them is difficult in part because African governments have not committed to buying African-made vaccines. African countries also need to strengthen their workforce and their ability to evaluate and approve vaccines, the report says.
Any African vaccine manufacturer will face stiff competition from vaccines produced elsewhere. The Serum Institute of India, which produces more vaccine doses than any other company, is already a major supplier to Gavi.
To avoid fragmentation of Africa’s budding market, the Regionalized Vaccine Manufacturing Collaborative, announced in January, aims to streamline and coordinate efforts. Ideally, countries in a region will pool resources, make bulk purchases of supplies, and evaluate demand together. Nkengasong says Africa may best be served by two or three vaccine manufacturing networks.