Congo to receive first mpox vaccines to address outbreak; The latest health stories from around the world
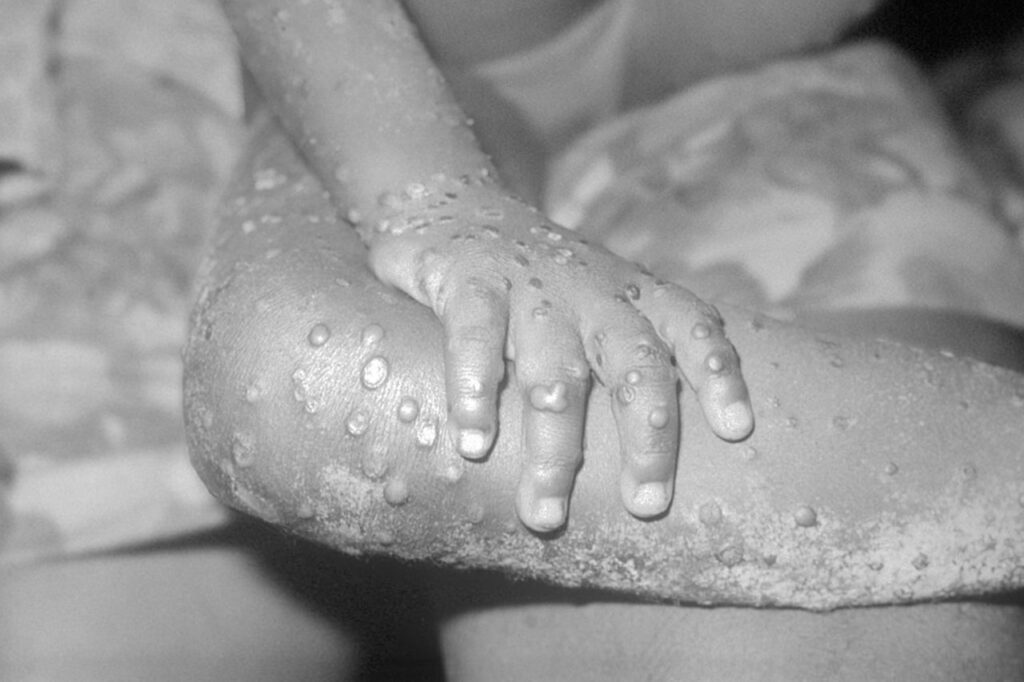
Article by Lalita Panicker, Consulting Editor, Views and Editor, Insight, Hindustan Times, New Delhi Congo will receive the first vaccine doses to address its mpox outbreak this week from the United States, the country’s health minister said last week, days after the World Health Organization declared mpox outbreaks in Africa a global emergency. https://apnews.com/article/congo-mpox-vaccine-united-states-emergency-8490a15ed4937bfab9477529302f0469 Mpox cases have been […]