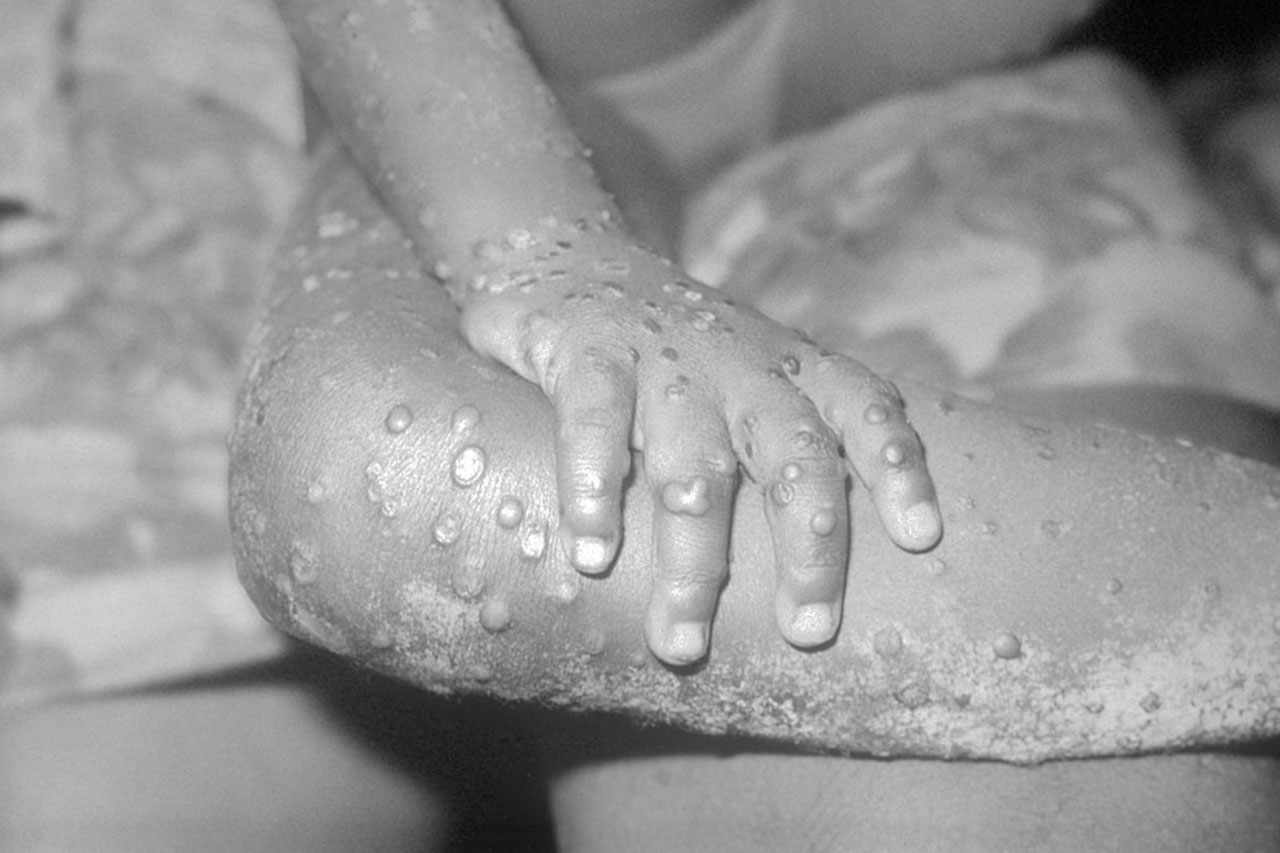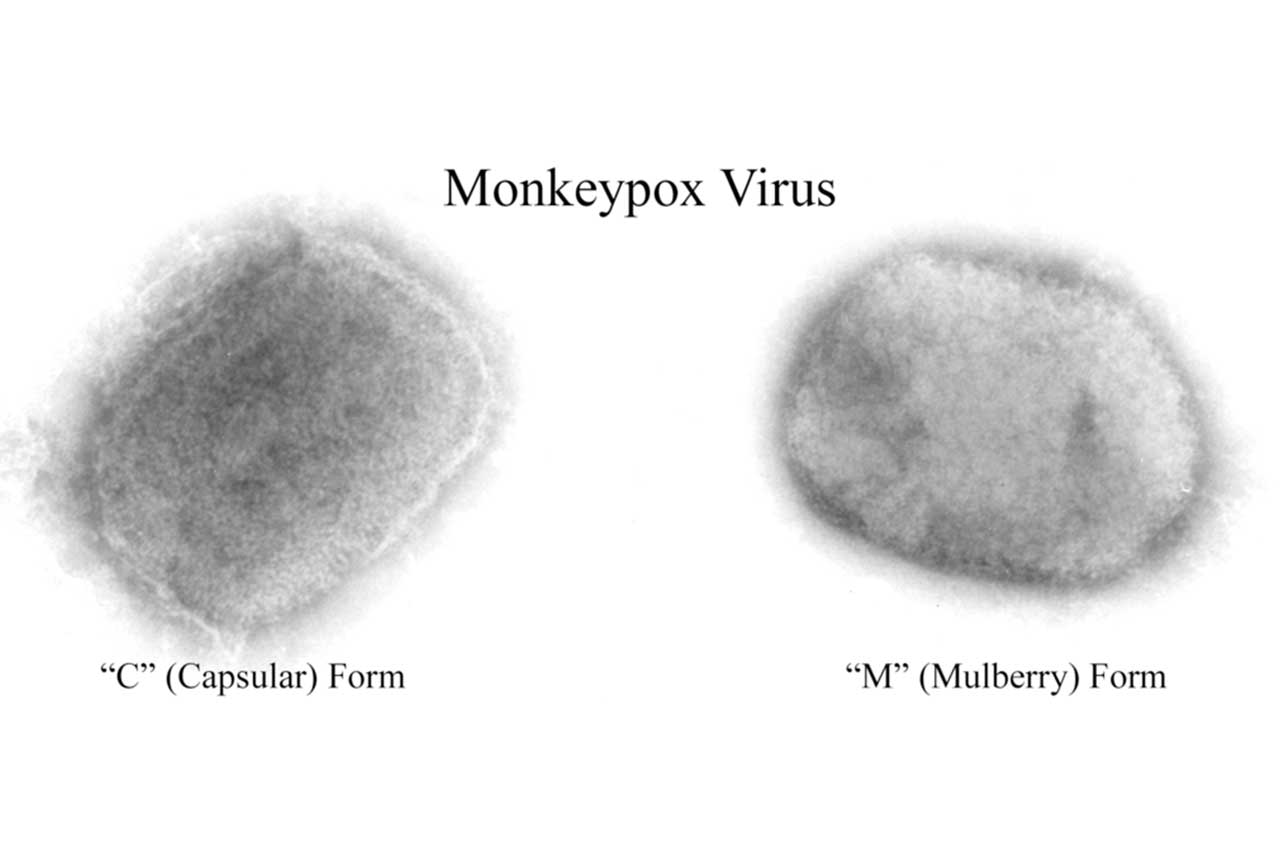
Article by Lalita Panicker, Consulting Editor, Views and Editor, Insight, Hindustan Times, New Delhi
The 2023 Nobel Prize in Physiology or Medicine has been jointly awarded to Katalin Karikó and Drew Weissman for their discoveries concerning nucleoside base modifications that enabled the development of effective mRNA vaccines against COVID-19 https://www.nobelprize.org/prizes/medicine/2023/press-release/
The discoveries by the two Nobel Laureates were critical for developing effective mRNA vaccines against COVID-19 during the pandemic that began in early 2020. Through their ground breaking findings, which have fundamentally changed our understanding of how mRNA interacts with our immune system, the Laureates contributed to the unprecedented rate of vaccine development during one of the greatest threats to human health in modern times.
Katalin Karikó was born in 1955 in Szolnok, Hungary. She received her PhD from Szeged’s University in 1982 and undertook postdoctoral research at the Hungarian Academy of Sciences in Szeged until 1985. She then conducted her postdoctoral research at Temple University, Philadelphia, and the University of Health Science, Bethesda. In 1989, she was appointed Assistant Professor at the University of Pennsylvania, where she remained until 2013. After that, she became vice president and later senior vice president at BioNTech RNA Pharmaceuticals. Since 2021, she has been a Professor at Szeged University and an Adjunct Professor at Perelman School of Medicine at the University of Pennsylvania.
Drew Weissman was born in 1959 in Lexington, Massachusetts, USA. He received his MD, PhD degrees from Boston University in 1987. He did his clinical training at Beth Israel Deaconess Medical Center at Harvard Medical School and postdoctoral research at the National Institutes of Health. In 1997, Weissman established his research group at the Perelman School of Medicine at the University of Pennsylvania. He is the Roberts Family Professor in Vaccine Research and Director of the Penn Institute for RNA Innovations.
/////
The World Health Organization (WHO) has recommended a new vaccine, R21/Matrix-M, for the prevention of malaria in children. The recommendation follows advice from the WHO: Strategic Advisory Group of Experts on Immunization (SAGE) and the Malaria Policy Advisory Group (MPAG) and was endorsed by the WHO Director-General following its regular biannual meeting held on 25-29 September. https://www.who.int/news/item/02-10-2023-who-recommends-r21-matrix-m-vaccine-for-malaria-prevention-in-updated-advice-on-immunization
WHO also issued recommendations on the advice of SAGE for new vaccines for dengue and meningitis, along with immunisation schedule and product recommendations for COVID-19. WHO also issued key immunization programmatic recommendations on polio, IA2030 and recovering the immunization programme.
The R21 vaccine is the second malaria vaccine recommended by WHO, following the RTS,S/AS01 vaccine, which received a WHO recommendation in 2021. Both vaccines are shown to be safe and effective in preventing malaria in children and, when implemented broadly, are expected to have high public health impact. Malaria, a mosquito-borne disease, places a particularly high burden on children in the African region, where nearly half a million children die from the disease each year.
Demand for malaria vaccines is unprecedented; however, available supply of RTS,S is limited. RTS,S or Mosquirix, was recommended for use in 2021 and has been given to 1.8 million children in Ghana, Malawi, and Kenya. Doses are expected to arrive in nine more countries in Africa by the end of the year. But the 18 million doses of that vaccine that are expected to be available between now and 2025 “are only about 10% of what we need” to protect the estimated 40 million children who are born every year in malaria-affected areas, says Matthew Laurens, a malaria vaccine expert at the University of Maryland School of Medicine. R21 could soon be available in much larger quantities.
The addition of R21 to the list of WHO-recommended malaria vaccines is expected to result in sufficient vaccine supply to benefit all children living in areas where malaria is a public health risk.
“As a malaria researcher, I used to dream of the day we would have a safe and effective vaccine against malaria. Now we have two,” said Dr Tedros Adhanom Ghebreyesus, WHO Director-General. “Demand for the RTS,S vaccine far exceeds supply, so this second vaccine is a vital additional tool to protect more children faster, and to bring us closer to our vision of a malaria-free future.”
The updated WHO malaria vaccine recommendation is informed by evidence from an ongoing R21 vaccine clinical trial and other studies, which showed:
- High efficacy when given just before the high transmission season: In areas with highly seasonal malaria transmission (where malaria transmission is largely limited to 4 or 5 months per year), the R21 vaccine was shown to reduce symptomatic cases of malaria by 75% during the 12 months following a 3-dose series. A fourth dose given a year after the third maintained efficacy. This high efficacy is similar to the efficacy demonstrated when RTS,S is given seasonally.
- Good efficacy when given in an age-based schedule: The vaccine showed good efficacy (66%) during the 12 months following the first 3 doses. A fourth dose a year after the third maintained efficacy.
- High impact: Mathematical modelling estimates indicate the public health impact of the R21 vaccine is expected to be high in a wide range of malaria transmission settings, including low transmission settings.
- Cost effectiveness: At prices of US$ 2 – US$ 4 per dose, the cost-effectiveness of the R21 vaccine would be comparable with other recommended malaria interventions and other childhood vaccines.
- Similarity of R21 and RTS,S vaccines: The two WHO-recommended vaccines, R21 and RTS,S, have not been tested in a head-to-head trial. There is no evidence to date showing one vaccine performs better than the other. The choice of product to be used in a country should be based on programmatic characteristics, vaccine supply, and vaccine affordability
- Safety: The R21 vaccine was shown to be safe in clinical trials. As with other new vaccines, safety monitoring will continue.
Next steps for the second recommended malaria vaccine, R21/Matrix-M, include completing the ongoing WHO prequalification which would enable international procurement of the vaccine for broader rollout.
Like RTS,S, R21 requires multiple initial doses and a later booster. Both vaccines induce immunity with a protein from the Plasmodium parasite that causes malaria. But the new vaccine contains a different immune-boosting agent, or adjuvant, which is somewhat easier to produce than the one used in RTS,S. The vaccine’s developers have already worked with the Serum Institute of India (SII), one of the world’s biggest vaccine makers, to manufacture R21. SII says it can produce more than 100 million doses of the vaccine per year. That would be “a leap forward” for efforts to fight malaria, Laurens says. “Having a second product would be fabulous.” And at an estimated price of less than $5 per dose, the new vaccine may be less expensive than RTS,S, which cost €9.30 per dose in the first major purchase of the vaccine by UNICEF.
The preprint now reports that at the three trial sites where malaria transmission is seasonal, the vaccine had 75% efficacy over 18 months compared with preventive drugs alone. At the two sites with year-round transmission, the efficacy was 68%. www.science.org/content/article/promising-malaria-vaccine-passes-final-clinical-test-could-get-who-approval-next-week?
At least 28 countries in Africa plan to introduce a WHO-recommended malaria vaccine as part of their national immunisation programmes. Gavi, the Vaccine Alliance has approved providing technical and financial support to roll out malaria vaccines to 18 countries. The RTS,S vaccine will be rolled out in some African countries in early 2024, and the R21 malaria vaccine is expected to become available to countries mid-2024.
Recommendations on dengue
- The live-attenuated quadrivalent dengue vaccine developed by Takeda (TAK-003) has demonstrated efficacy against all four serotypes of the virus in baseline seropositive children (4-16 years) in endemic countries and against serotypes 1 and 2 in baseline seronegative children.
- SAGE recommended that the vaccine be considered for introduction in settings with high dengue disease burden and high transmission intensity to maximize the public health impact and minimize any potential risk in seronegative persons.
- SAGE recommended that the vaccine be introduced to children aged 6 to 16 years of age. Within this age range, the vaccine should be introduced about 1-2 years prior to the age-specific peak incidence of dengue-related hospitalizations. The vaccine should be administered in a 2-dose schedule with a 3-month interval between doses.
////
In a first for Europe, France plans to start vaccinating poultry next week against highly pathogenic avian influenza (HPAI) viruses. The compulsory inoculations will begin at commercial duck farms, French agricultural officials said, and are expected to be expanded to other poultry. www.science.org/content/article/news-glance-china-s-s-t-clusters-abundant-fairy-circles-and-arecibo-s-next-chapter?
The effort aims to stop a variant of the flu virus that has devastated the continent’s poultry industry since 2020 and killed many wild birds and sea mammals around the world. Europe has not previously used vaccines against HPAI outbreaks because of trade concerns; in theory, vaccination could mask symptoms of infections, leading to unwitting importation of the highly lethal virus. Instead, European countries combated HPAI outbreaks by culling infected flocks and stepping up biosecurity. But those methods have not stopped this flu variant.
////
Scientists have identified two types of brain cell linked to a reduced risk of dementia — even in people who have lots of the amyloid ‘plaques’ that are hallmarks of Alzheimer’s disease. The neurons might have a role in the types of cognitive function that are lost during Alzheimer’s, hinting at future treatments that focus on protecting them.
////
A little suction cup could deliver medication straight into the bloodstream without an injection. Inspired by octopuses — who know something about grabbing onto wet bits — researchers designed the cup to stick to the inside of a person’s cheek. It painlessly grabs and stretches the inner mouth tissue so that a drug, stored inside, can enter the blood. Most people who tested the device preferred using it to being injected with a needle. “The biggest problem with insulin is the degradation in the gastrointestinal tract, which can be minimized thanks to this suction patch,” says clinical pharmacist Simon Matoori. (Nature | 4 min read)
Reference: Science Translational Medicine paper (ETH Zurich)
////
When the World Health Organization called attention in 2022 to a surge in cholera outbreaks around the globe, it listed the usual factors that favour waterborne diseases: lack of sanitation, humanitarian crises, and conflict. But it also said climate change was worsening the situation. https://www.science.org/doi/10.1126/science.adl0414?
Cholera is caused by Vibrio cholerae, a bacterium that can produce a toxin and spreads when the stool of infected people contaminates drinking water and food. Microbiologist Rita Colwell has long argued that warmer surface waters can favour the emergence of the bacterium. Whether this plays any role in the large outbreaks around the globe is contested. But another climate link is clear. Floods can aid spread by causing latrines to overflow into water sources, for instance, and droughts can boost the concentration of the bacterium in shrinking ponds and streams and force people to use unsafe water.
Climate change is making such extreme weather events more frequent, so cholera is likely to surge in a warming world, says Andrew Azman, an epidemiologist at Johns Hopkins University. But there is little consensus on the magnitude of the effect.