Barney Graham, a former scientist at the U.S. National Institute of Allergy and Infectious Diseases (NIAID), was thrilled when Pfizer announced encouraging results from an experimental vaccine that could protect against a major childhood killer. In a press release, the company said immunizing pregnant women with its vaccine against respiratory syncytial virus (RSV) protected their babies from severe disease for 6 months. www.science.org/content/article/extremely-satisfying-scientists-insight-powers-new-rsv-vaccine-infants?
If the full results of its clinical trial bear out that promise, the vaccine could spare millions of infants worldwide from RSV-related hospitalization, reduce lasting lung damage from the virus, and help prevent some of the estimated 120,000 childhood deaths the viruses cause annually. The Pfizer news represents a personal milestone for Graham because his NIAID team made a fundamental discovery about vaccine design that’s used in its RSV shot, as well as in approved or potential vaccines for COVID-19 and other infectious diseases.
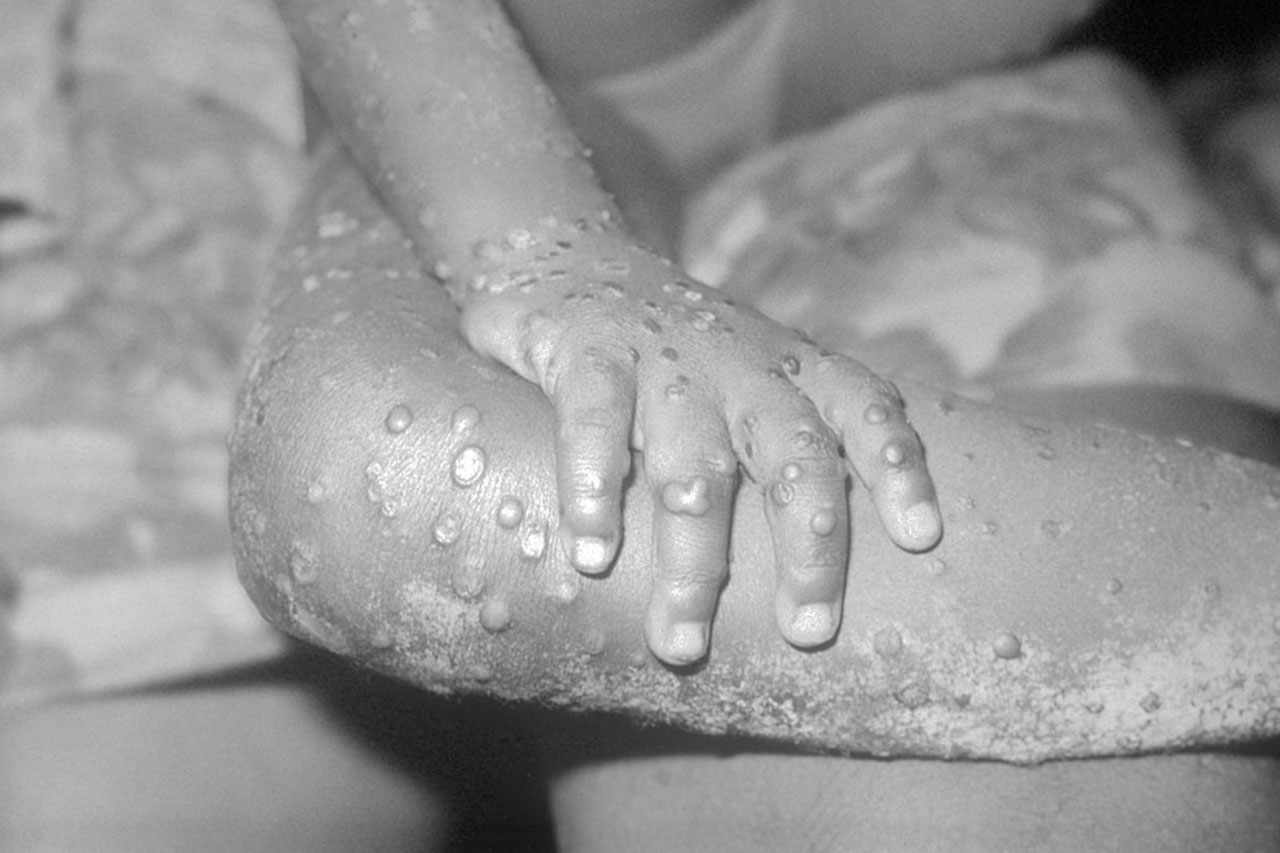
Vaccine development for RSV was derailed after a trial of a candidate in 1966 led to two deaths and the hospitalization of 80 percent of the infants who received the product, which contained an inactivated version of the entire virus. But Graham, who began working on RSV in 1985 when he was a 32-year-old clinician, created a safer, more potent vaccine that only contains the RSV surface protein known as F.
The F protein rearranges its structure when the virus infects and fuses with a cell. Graham, who is now at the Morehouse School of Medicine, led an NIAID team that designed a molecular strategy to lock F into its original, “prefusion” configuration, stimulating the most powerful antibody responses. Graham’s NIAID group made a similar modification to the SARS-CoV-2 surface protein, spike, which was adopted by Moderna, Pfizer, and other companies in their designs of COVID-19 vaccines.
In Pfizer’s trial, which is taking place in 18 countries, 7,400 pregnant people received one dose of “RSVpreF” or a placebo shot during their late second or third trimester. Mothers naturally transfer the RSV antibodies stimulated by the vaccine to their foetuses. An independent committee monitoring the data recommended the trial stop enrolling people because the study had met one of two of its primary endpoints, but Pfizer will continue to track their infants, some 7,000 so far.
A recent study of the global impact of RSV infections in older adults estimated it leads to 336,000 hospitalizations and 14,000 in-hospital deaths.