Article by Lalita Panicker, Consulting Editor, Views and Editor, Insight, Hindustan Times, New Delhi
Chinese researchers released a trove of new genetic data last Wednesday that may offer fresh clues to the origin of the COVID-19 pandemic. They also substantially revised a related study they first posted online 13 months ago to include this evidence, which some scientists say gives more credibility to the thesis that SARS-CoV-2 could have jumped into humans from raccoon dogs or other mammals illegally sold at a Wuhan market. www.science.org/content/article/chinese-researchers-release-genomic-data-help-clarify-origin-covid-19-pandemic?
The Chinese team’s initial preprint argued that the market data, consisting of genetic sequences found in 923 samples collected in or near the market in early 2020, “highly suggests” humans brought the coronavirus there–and made no mention of evidence showing that SARS-CoV-2 susceptible mammals were present. Their updated preprint acknowledges the genetic evidence of the animals and now says the collected samples don’t resolve whether infected animals or humans, or even contaminated food, introduced the virus into the market, where the first cluster of COVID-19 cases surfaced.
“I’m pleased that the data have been updated and made available on different platforms and that [the researchers] have made their updated manuscript available on a preprint server,” says Maria Van Kerkhove, an epidemiologist at the World Health Organization (WHO) who last week urged the Chinese group to share the market data after another research team stumbled on some of it.
Written by researchers primarily affiliated with the Chinese Center for Disease Control and Prevention (CCDC), the preprint focuses on “environmental samples”—from drains, containers, tables, doors, the ground—that the authors took at the Huanan Seafood Wholesale Market in January to March of 2020. After COVID-19 cases began popping up there in December 2019, the market was quickly shut down by Chinese authorities on 1 January 2020. Initially, CCDC researchers, including preprint co-author George Gao (who then headed the agency), said they suspected animals at the market triggered the outbreak. But they later denied that the market sold illegal mammals, arguing that imported fish or people from other countries brought the virus there.
Now Gao and his co-authors have made a great deal more of their data available on databases including GISAID, which many researchers use to share genetic sequences of SARS-CoV-2. In their revised preprint, which they posted on 29 March on the server ChinaXiv, they note that “environmental samples showed the abundance of different vertebrata genera.” Some of these same samples also had traces of SARS-CoV-2. However, “these environmental samples cannot prove the infection of the animals,” caution Gao, William Liu, Guizhen Wu, and their co-authors.
“Furthermore, even if the animals were infected, it could not still rule out that the human-to-animal transmission occurred, considering the sampling time was at least one month after the human-to-human transmission within the market. Thus, the possibility of potential introduction of the virus through human or cold chain product into the market cannot yet be ruled out,” they conclude.
The genetic sequences from the Wuhan market took an unusual journey into the spotlight. Florence Débarre, an evolutionary biologist at the French national research agency, CNRS, was surprised to find a subset of the data 3 weeks ago while combing through GISAID. When she collaborated with a team of scientists from outside of China to analyse the sequences, they found the evidence that the market had the mammals, which they took to WHO. The finding first became public on 16 March in an Atlantic story titled “The Strongest Evidence Yet That an Animal Started the Pandemic.”
Explaining that they did not want to scoop a journal publication by Chinese researchers, Débarre and her colleagues posted a report online on 20 March that analysed the data but did not provide the sequences. GISAID temporarily suspended their access, asserting that the team had violated the database’s terms of access. But GISAID restored the access when the group provided evidence of having offered to collaborate with the Chinese researchers.
/////
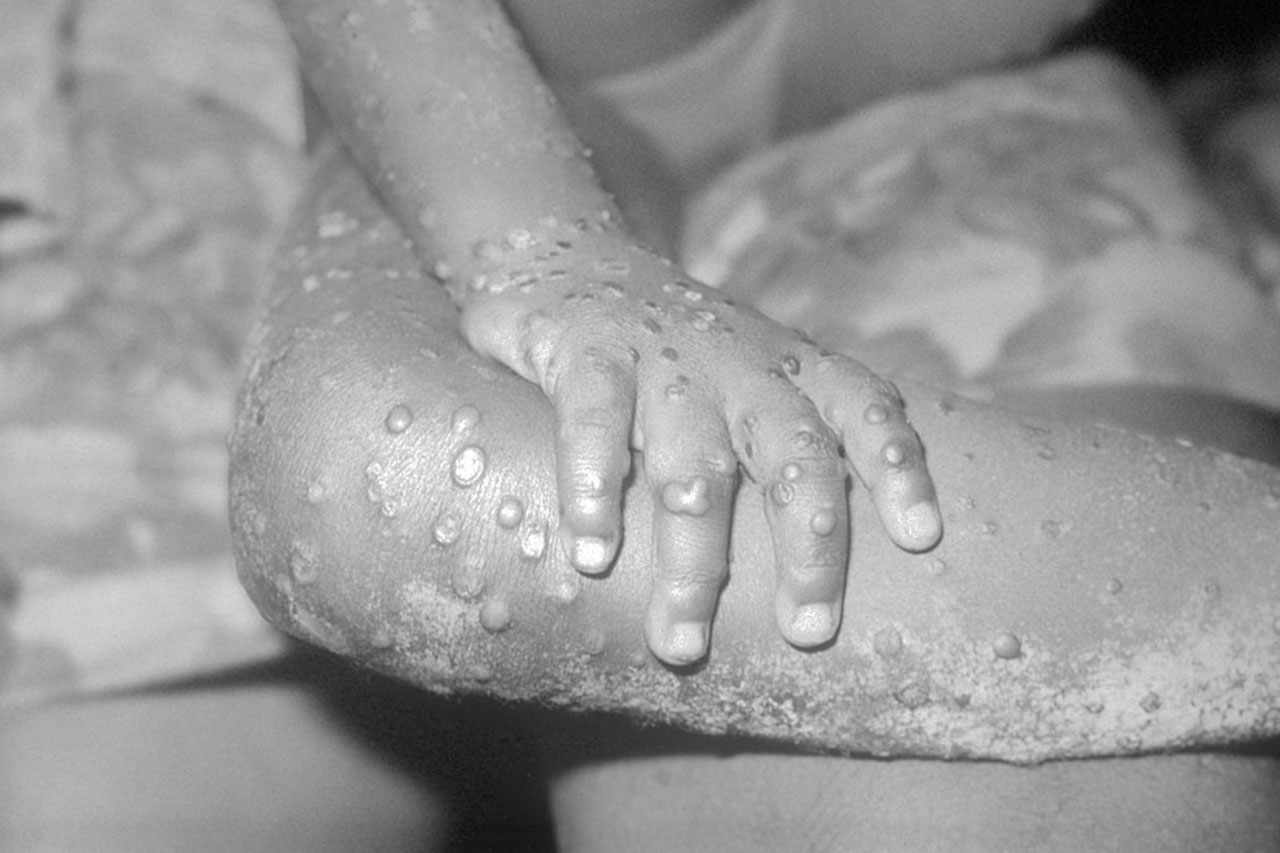
Public health experts are combating two outbreaks of the deadly Marburg virus on opposite sides of the African continent. Authorities in Equatorial Guinea have reported nine confirmed and 20 probable cases of the haemorrhagic fever since early January; 27 of the 29 patients have died. https://www.science.org/content/article/news-glance-particles-weighty-measurement-marburg-africa-fossil-called-blob?
The cases are spread across different provinces, and health officials say the lack of connection between some cases suggests there is undetected spread in the community. In Tanzania, eight cases have been reported, including five deaths. This is the first Marburg outbreak in each country, and genome sequencing is ongoing to determine whether the two outbreaks are related. The risk of spread to other countries in the region is also high, the World Health Organization has warned. Unlike Ebola disease, to which it is related, Marburg has no approved vaccines or antivirals.
////
China last week approved for emergency use its first COVID-19 vaccine using messenger RNA (mRNA) technology. www.science.org/content/article/news-glance-particles-weighty-measurement-marburg-africa-fossil-called-blob?
The homegrown product, developed by CSPC Pharmaceutical Group, comes about 2 years after much of the rest of the world began to get shots based on the novel vaccine platform, developed in the United States and Europe. Trials with more than 5500 participants showed the vaccine is safe and effective, CSPC reported. China’s Shanghai Fosun Pharmaceutical in March 2020 obtained the rights to market BioNTech’s mRNA COVID-19 vaccine in China once it was developed, but regulators never approved it. China has instead relied on less effective, traditional vaccines made with inactivated coronaviruses.
////
Drugs that combat obesity are under consideration for the first time for the World Health Organization’s “essential medicines list,” used to guide government purchasing decisions in low- and middle-income countries, the UN agency told Reuters. www.reuters.com/business/healthcare-pharmaceuticals/who-consider-adding-obesity-drugs-essential-medicines-list-2023-03-29/
A panel of advisers to the WHO will review new requests for drugs to be included next month, with an updated essential medicines list due in September.
The request to consider obesity drugs was submitted by three doctors and a researcher in the United States. It covers the active ingredient liraglutide in Novo Nordisk’s (NOVOb.CO) obesity drug Saxenda, which will come off patent soon, allowing for cheaper generic versions.
The panel could reject the request or wait for more evidence. A decision by the WHO to include Saxenda and eventual generics on the list for adults would mark a new approach to global obesity by the health agency.
It could also pave the way for a newer, more powerful treatment from Novo Nordisk called Wegovy to be recommended for low- and middle-income countries in future.
However, some public health experts warn against introducing such medicines too broadly as a solution to a complex condition that is still not completely understood.
“We believe it is a work in progress,” said Francesco Branca, WHO director of nutrition, at a press briefing on 29 March, referring to the use of drugs as obesity treatments.
He said there were still issues around the cost of liraglutide as well as the fact that it had not been in use long enough which may make inclusion on the list unlikely, but it was up to the expert committee to review the evidence and decide.
“At the same time, WHO is looking at the use of drugs to reduce weight … in the context of a systematic review for guidelines for children and adolescents,” he said.
Over 650 million adults worldwide are obese, more than triple the rate in 1975, and roughly another 1.3 billion are overweight, according to the WHO. The majority of obese and overweight people – 70% – live in low- and middle-income countries.
Including obesity drugs among the WHO’s essential medicines could have great significance for that population. Experts say that adding HIV drugs to the list in 2002 helped to make them much more widely available to AIDS patients in poorer countries.
“At present, there are no medications included in the (list) that specifically target weight loss for the ongoing global burden of obesity,” wrote US researcher Dr Sanjana Garimella from Yale New Haven Health, Dr Sandeep Kishore from the University of California, San Francisco, and colleagues to the WHO in requesting the addition.
They argue that while the list includes mineral supplements for nutritional deficiencies, the lack of weight-loss treatments represents a “discrepancy” in global health equity, given the increasing number of deaths in poorer nations hastened by weight-related illness, including heart disease and diabetes.
Saxenda, a once-daily injection, has been shown to help people reduce 5%-10% of their body weight, at $450 per month in the United States and $150 per month in Europe.
People using Wegovy, a weekly injection that costs more than $1,300 a month in the United States, have lost up to 15% of their weight. At the moment, Wegovy is in short supply and Novo is prioritizing its launch and distribution in the US and other wealthy markets.
The Danish drugmaker in a statement said it was not involved in the application to consider liraglutide for inclusion on the WHO list, adding, “we welcome the WHO review and look forward to the readout and decision.”
Both drugs belong to a class of medicines called GLP-1 receptor agonists, which have been used for years to treat diabetes. They affect hunger signals to the brain and slow the rate at which a person’s stomach empties, making them feel fuller longer. Eli Lilly and Co (LLY.N) has a similar diabetes drug nearing approval for weight loss.
For both Saxenda and Wegovy, there is a lack of long-term safety and effectiveness data for obesity. Studies suggest people will likely have to take the drugs for the rest of their lives to keep the weight off.
High-income countries are taking varying approaches for how to use these medicines, including contemplating whether they can be prescribed by government-sponsored health systems or covered by insurance, as they are for diabetes. In some countries, their use is being reserved only for the most at-risk groups.