Author CDC/ Cynthia S. Goldsmith, Inger K. Damon, and Sherif R. Zaki
A few years ago, researchers scoured the remains of 1867 people who lived between 30,000 and 150 years ago for genetic traces of variola, the virus that causes smallpox. In the teeth and bones of four Northern Europeans from the Viking era, they found enough DNA to reconstruct entire variola genomes. The sequenced viruses weren’t direct ancestors of the feared variola strain that was eradicated in the second half of the 20th century. But they may hold a clue to how smallpox became so deadly. www.science.org/content/article/will-monkeypox-virus-become-more-dangerous?utm_source=sfmc
Over the span of 350 years, the Viking virus lost several genes, the researchers reported in a 2020 paper in Science. Researchers had seen this pattern before. The modern smallpox virus also lost several genes in the recent past, although as a result of different mutations. Seeing it twice “suggests that the loss of the genes was not an accident,” says pox virologist Antonio Alcamí of the Sivero Ochoa Centre of Molecular Biology in Madrid. Alcamí thinks the losses may have made variola more virulent, resulting in its 30% mortality rate. In the past, smallpox may have been a “widespread mild disease,” he wrote in a commentary accompanying the paper.
Now, some scientists are wondering: Could something like this happen again?
Since May, a far less lethal cousin of variola, the monkeypox virus, has been spreading around the globe, giving the virus unprecedented opportunities to change and adapt to the human population. Will it evolve to become more contagious or cause more severe disease?
Nobody knows, but recent history with SARS-CoV-2 offers a sobering lesson. After emerging in Wuhan in late 2019, that virus first spawned a series of variants that could spread much faster than their progenitors and then evolved further to evade human immunity. Its tricks surprised even some scientists who have long studied viral evolution. SARS-CoV-2 showed that “if a novel virus is coming into a space in which there isn’t immunity, rapid adaptation can happen,” says Aris Katzourakis, an evolutionary virologist at the University of Oxford.
Monkeypox could present humanity with equally unpleasant surprises. In July, researchers in Berlin published a preprint analysing the genome sequences of virus found in the lesions of 47 monkeypox patients. In addition to many small changes, they found one virus in which an entire gene was duplicated and four others were simply gone. The paper’s last paragraph almost read like a warning: “The consequence of changes in poxvirus genes whose products are no longer required in a new host or otherwise altered context is unpredictable,” the authors said. “The [monkeypox virus] phenotype we have known for the last 64 years may not resemble near-future human” monkeypox.
Many researchers say we shouldn’t worry too much yet. Geoffrey Smith, a pox virologist at the University of Cambridge, doubts the monkeypox virus will readily turn into a much more virulent version. Poxviruses’ massive genomes are known to evolve at a sluggish pace, and they don’t adapt easily to elude immunity, as SARS-CoV-2 does so masterfully. And SARS-CoV-2 is a wildly contagious respiratory pathogen that infected hundreds of millions in its first year in the human population; monkeypox is spreading mostly among men who have sex with men, and only about 60,000 cases have been reported so far, so it has much less opportunity to evolve.
That could change, however. One “bad scenario,” says Bernard Moss, a veteran poxvirus researcher at the U.S. National Institute of Allergy and Infectious Diseases, is that the virus evolves to replicate faster in humans. That would allow it to infect more people, which would in turn speed up its evolution, potentially making it still more adept at infecting people.
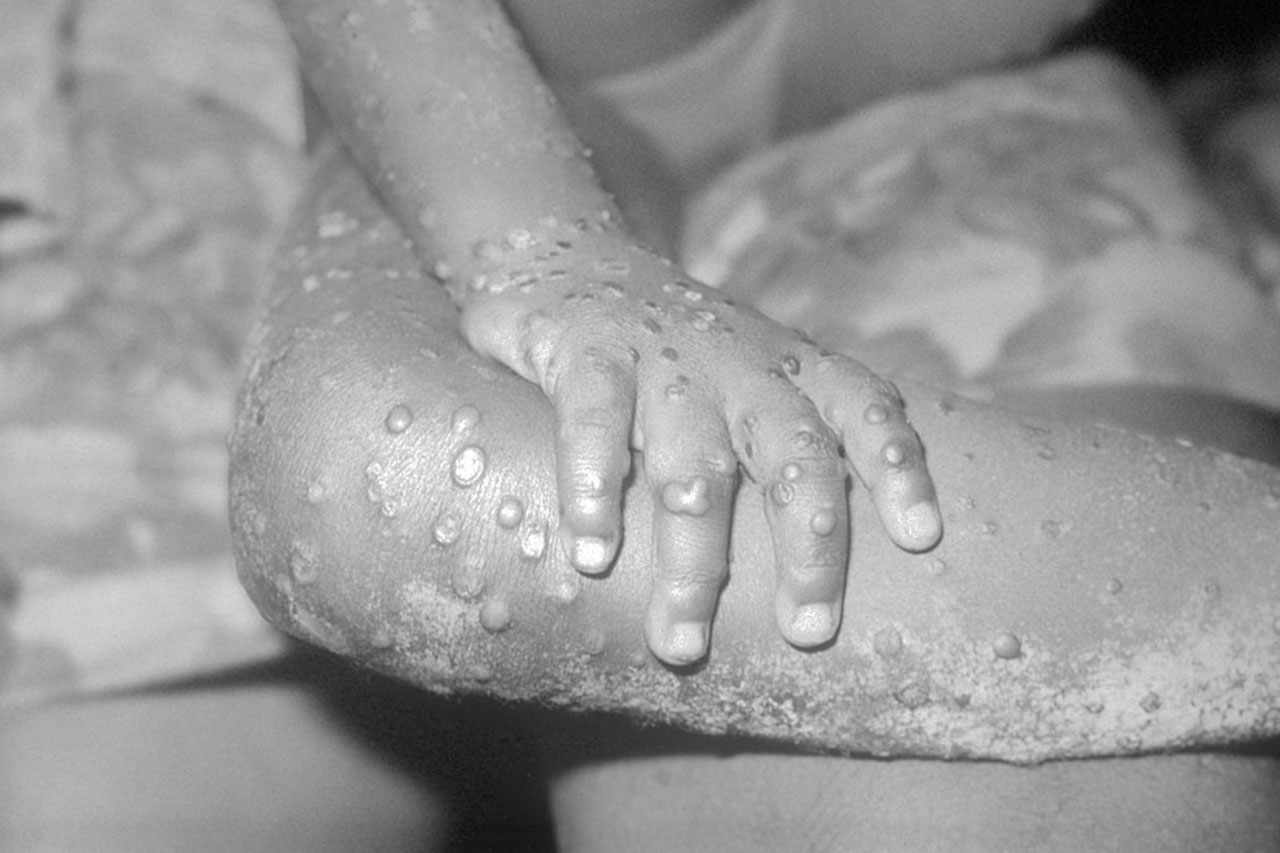
For now, the Monkeypox virus is not very good at infecting humans. It is a generalist that appears to thrive in a range of animal species—most of them rodents—in sub-Saharan Africa. From time to time the virus has spilled over into people, who have sometimes infected a few others. Although outbreaks have grown more frequent in recent years (see Perspective), they have typically been small. After each emergence the virus apparently disappeared again from the human population.
This time around is different, as monkeypox has continued to spread from person to person in a global outbreak. “We’ve never seen this virus with such an opportunity to adapt to humans before,” says Terry Jones, a computational biologist at Charita University Hospital in Berlin and one of the authors of the July preprint.
Reported cases are going down in many western countries—most likely as a result of behavioural changes and vaccination—and public health officials in Europe are already talking about eliminating the virus in the region. But infections are still on the rise elsewhere in the world. In many places vaccines are unavailable, or people at risk either lack information about how to avoid infection or fear asking for it, because gay sex is criminalized.
Researchers around the world are now mining monkeypox genomes from recent patients to learn how the virus has evolved so far. Getting high-quality sequences is harder and more expensive than it is for SARS-CoV-2, not just because the monkeypox genome is so vast but also because crucial regions near its ends can be full of repetitions or deletions that can trip up researchers when they assemble sequences. “Handling these genomes is more complex than the RNA viruses,” says Richard Neher, a computational biologist at the University of Basel. “It will be more important than with SARS-CoV-2 that people share their raw data.”