Article by Lalita Panicker, Consulting Editor, Views and Editor, Insight, Hindustan Times, New Delhi
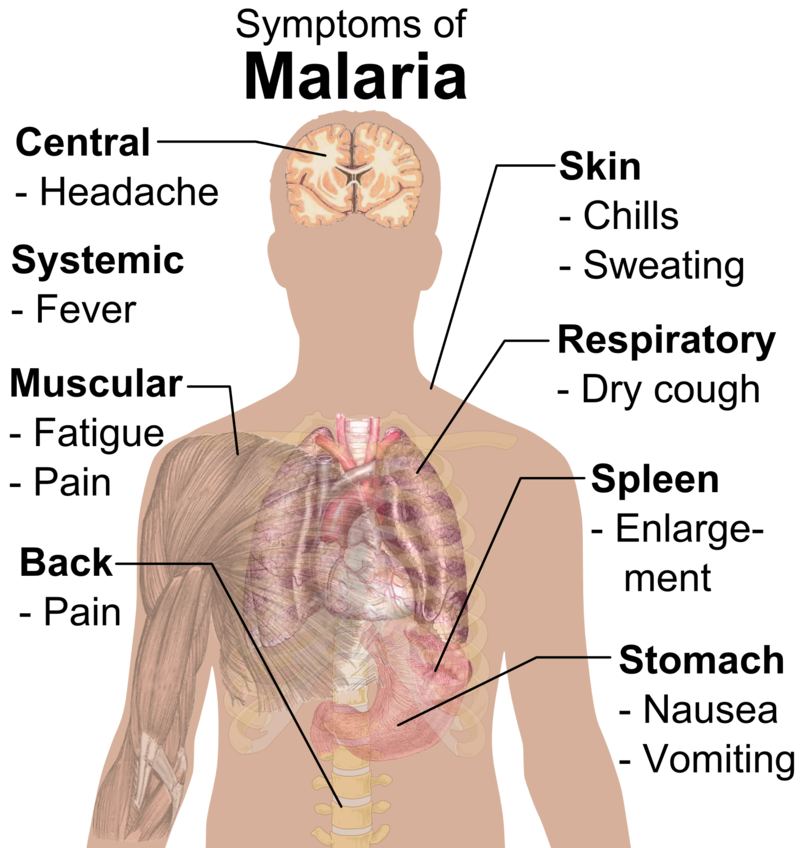
Twelve countries across different regions in Africa are set to receive 18 million doses of the first-ever malaria vaccine over the next two years. The roll out is a critical step forward in the fight against one of the leading causes of death in the continent. www.gavi.org/news/media-room/18-million-doses-first-ever-malaria-vaccine-allocated-12-african-countries-2023
The allocations have been determined through the application of the principles outlined in the Framework for allocation of limited malaria vaccine supply that prioritizes those doses to areas of highest need, where the risk of malaria illness and death among children are highest.
Since 2019, Ghana, Kenya and Malawi have been delivering the malaria vaccine through the Malaria Vaccine Implementation Programme (MVIP), coordinated by WHO and funded by Gavi, the Vaccine Alliance, the Global Fund to Fight AIDS, Tuberculosis and Malaria, and Unitaid. The RTS,S/AS01 vaccine has been administered to more than 1.7 million children in Ghana, Kenya and Malawi since 2019 and has been shown to be safe and effective, resulting in a substantial reduction in severe malaria and a fall in child deaths. At least 28 African countries have expressed interest in receiving the malaria vaccine.
In addition to Ghana, Kenya and Malawi, the initial 18 million dose allocation will enable nine more countries, including Benin, Burkina Faso, Burundi, Cameroon, the Democratic Republic of the Congo,
Liberia, Niger, Sierra Leone and Uganda, to introduce the vaccine into their routine immunisation programmes for the first time. This allocation round makes use of the supply of vaccine doses available to Gavi, Vaccine Alliance via UNICEF. The first doses of the vaccine are expected to arrive in countries during the last quarter of 2023, with countries starting to roll them out by early 2024.
Malaria remains one of Africa’s deadliest diseases, killing nearly half a million children each year under the age of 5, with Africa accounting for approximately 95% of global malaria cases and 96% of deaths in 2021.
“Nearly every minute, a child under 5 years old dies of malaria,” said UNICEF Associate Director of Immunization Ephrem T Lemango. “For a long time, these deaths have been preventable and treatable; but the roll-out of this vaccine will give children, especially in Africa, an even better chance at surviving. As supply increases, we hope even more children can benefit from this life-saving advancement.”
Annual global demand for malaria vaccines is estimated at 40–60 million doses by 2026 alone, growing to 80–100 million doses each year by 2030. In addition to the RTS,S/AS01 vaccine, developed and produced by GSK, and in the future supplied by Bharat Biotech, it is expected that a second vaccine, R21/Matrix-M, developed by Oxford University and manufactured by Serum Institute of India (SII), could also be prequalified by WHO soon. Gavi has recently outlined its roadmap to support increasing supply to meet demand.
////
The US Food and Drug Administration (FDA) has fully approved the first drug shown to slow down Alzheimer’s disease. www.npr.org/sections/health-shots/2023/07/06/1186225580/alzheimers-drug-leqembi-gets-full-fda-approval-medicare-coverage-lecanemab?
The action means that Leqembi, whose generic name is lecanemab, should be widely covered by the federal Medicare health insurance program, which primarily serves adults age 65 and older. So more people who are in the early stages of the disease will have access to the drug – and be able to afford it.
“It’s not something that’s going to stop the disease or reverse it,” says Dr. Sanjeev Vaishnavi, director of clinical research at the Penn Memory Centre. “But it may slow down progression of the disease and may give people more meaningful time with their families.”
In studies reviewed by the FDA, Leqembi appeared to slow declines in memory and thinking by about 27% after 18 months of treatment. It also dramatically reduced the sticky beta-amyloid plaques that tend to build up in the brains of people with Alzheimer’s.
“It’s very exciting that we’re targeting the actual pathology of the disease,” Vaishnavi says.
Just to be talking about a treatment “is an incredible point for the Alzheimer’s cause overall,” says Joanne Pike, president and CEO of the Alzheimer’s Association.
Leqembi comes from the Japanese pharmaceutical company Eisai and its U.S. partner Biogen. The companies have said Leqembi will cost about $26,500 a year. In January, the drug received what’s known as accelerated approval from the FDA, based on its ability to remove the substance beta-amyloid from the brains of people in the early stages of Alzheimer’s. Full or traditional approval reflects the FDA’s assessment that Leqembi also helps preserve memory and thinking.
Also in January, the Centres for Medicare and Medicaid Services announced it would broaden coverage of Leqembi on the same day the drug received full FDA approval. That should mean the drug will now be covered for most Medicare patients with early signs of cognitive problems and elevated levels of amyloid.
Until now, Medicare has paid for Leqembi only for patients in certain clinical trials.
Under the expanded coverage, a million or more Medicare patients are potential candidates for the drug. But it’s likely that a much smaller number will actually get it in the next year or so.
One reason is the drug’s potentially life-threatening side effects, Vaishnavi says.
“I think [patients] are a little wary because they hear about bleeding or swelling in the brain,” Vaishnavi says. “They are concerned, and I think rightfully so.”
////////
Novavax Inc (NVAX.O) said on Friday Canada will pay $349.6 million to settle the forfeiting of certain doses of its COVID-19 vaccine previously scheduled for delivery. www.reuters.com/business/healthcare-pharmaceuticals/novavax-receive-350-mln-canada-unused-covid-shots-2023-07-07/#:~:text=July%207%20(Reuters)%20%2D%20Novavax,vaccine%20previously%20scheduled%20for%20delivery.
The U.S. vaccine maker also reached a deal with the country’s public works and government services department to amend the advance purchase contract after a sharp decline in global demand left a raft of COVID-19 doses unused.
The number of vaccine doses due for delivery has been reduced and the schedule for the remaining doses to be shipped revised under the amended terms, the company said.
The payment will be made in two equal instalments in 2023 and the original value of the contract remains unchanged.
However, the department can terminate the contract if the company fails to achieve regulatory approval for vaccine production at the Biologics Manufacturing Centre by Dec. 31, 2024.
Novovax – which has its COVID-19 vaccine as the only marketed product after 35 years in business – has raised doubts about its ability to remain in business, flagging uncertainties around its revenue and funding crunch.
The company said in May it expects 2023 revenue between $1.4 billion and $1.6 billion, of which $800 million was from “locked-in” overseas purchase contracts for the COVID shot that it has committed to ship this year.