Article by Lalita Panicker, Consulting Editor, Views and Editor, Insight, Hindustan Times, New Delhi
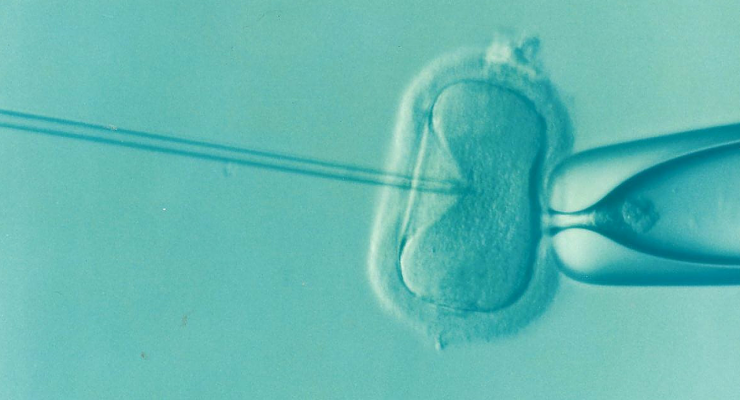
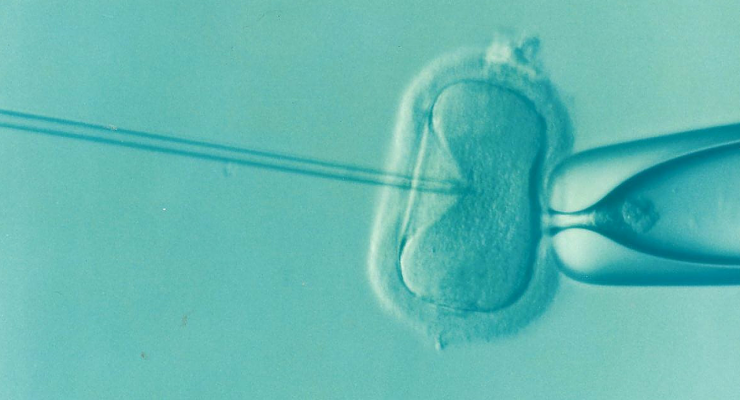
The Supreme Court of Alabama’s ruling last week declaring frozen embryos at fertility clinics to be people has upended patient care there. The state’s two leading private in vitro fertilization providers as well as the University of Alabama at Birmingham (UAB) paused all IVF procedures earlier this week while officials sort through the legal risks of continuing to create and store embryos and impregnate patients. https://www.science.org/content/article/alabama-ivf-ruling-may-halt-uterine-transplant-program?
But the impacts extend beyond would-be parents, to research. A uterus transplant program in which women conceive by IVF—one of only four in the country—is located at UAB, and its leader is also co–principal investigator on a related project studying uterine immune cells. If other states follow Alabama’s lead, other research, including efforts to improve IVF outcomes and probe developmental biology, could be imperilled around the country. “There’s research that is being done not just on uterus transplants, but on IVF, on eggs, and on embryos,” says Kate O’Neill, a reproductive medicine physician who directs the Uterus Transplant Program at the University of Pennsylvania (UPenn). Such studies, she says, “are going to advance science, and if this spreads, [they] would be very difficult to do.”
In its 8-1 decision last week, the Alabama Supreme Court overturned a lower court decision denying several parents the ability to recover punitive damages for the accidental loss of their frozen embryos. They sued a fertility clinic—the Centre for Reproductive Medicine—and an affiliated medical centre in Mobile because their embryos, created through IVF, were dropped on the floor and rendered unviable. The lower court had held that frozen embryos were not children under a 150-year-old law that allows parents of a dead child to bring civil suits. The state Supreme Court, however, declared “the law applies to all unborn children, regardless of their location.”
And although the future of the ruling in Alabama is still uncertain—at least one state lawmaker is working on a bill to clarify that embryos are not “people” until they are implanted in the uterus and a viable pregnancy can be detected—the impacts on would-be parents in Alabama in the meantime were immediate. Many IVF procedures have been halted in a state where, in 2021, 1600 rounds of IVF treatment were completed to lead to 400 babies. But it also stands to affect UAB’s uterine transplant program, led by immunologist and transplant surgeon Paige Porrett, which aims to help people who were born with defective or absent uteruses or had hysterectomies bear children.
For women to qualify for a transplant, they must first have embryos frozen for implantation—an impossibility at UAB since Wednesday, when the university paused such IVF procedures. “Thanks to the Supreme Court ruling on Friday everything is at a full stop,” Elizabeth Goldman, a UAB transplant patient, wrote on Facebook. Goldman was born without a uterus and received a uterus transplant at UAB in 2022 after banking frozen embryos; she gave birth to a daughter in November 2023 and had been scheduled to have another embryo transferred in the coming months. “I’m on a timeline with uterus transplant and this just makes things way more complicated.”
UAB, which has touted the transplant program, declined to answer questions about it or to make Porrett, who heads the program, available for an interview. Porrett herself did not respond to an emailed interview request.
//////
The U.S. Food and Drug Administration last week approved an injection that treats severe frostbite in adults, lowering the risk of finger or toe amputation. The drug, iloprost, is marketed by Eicos Sciences as Aurlumyn. https://www.science.org/content/article/news-glance-protecting-queen-cell-therapy-solid-tumors-and-uv-telescope?
Physicians have struggled to effectively treat severe frostbite, which stops blood flow in the skin and underlying tissue of the extremities. Standard therapy has included blood thinners not approved to treat frostbite. Iloprost, originally approved in 2004 to treat pulmonary arterial hypertension, acts by dilating blood vessels and preventing clotting. In a clinical trial published in 2011, none of 16 participants with severe frostbite who received only iloprost had a bone-scan result indicating they would need amputation, versus nine of 14 who received no iloprost.
////
Researchers have discovered a potent antibody that can neutralize a key type of neurotoxin produced by four different deadly snake species from South Asia, Southeast Asia, and Africa—a step toward an antivenom that could be used on any of the 200 or so dangerous venomous snakes throughout the world. www.science.org/content/article/powerful-new-antivenom-raises-hopes-universal-solution-lethal-snakebites?
“We are wiping out a major subclass of neurotoxins here,” says Nicholas Casewell, a toxinologist at the Liverpool School of Tropical Medicine and co-author on a paper describing the antibody published today in Science Translational Medicine. “I think this is a really huge step in terms of what can be achieved by a single antibody.”
Snake venoms are a mix of dozens or even hundreds of compounds that target nerve cells, blood clotting, or tissues, killing an estimated 81,000 to 138,000 people around the globe annually and disabling hundreds of thousands more. The standard treatment is antivenoms, a cocktail of antibodies harvested from horses or sheep injected with nonlethal doses of the venom. Although these drugs save lives, “antivenoms suffer from numerous problems,” says Kartik Sunagar, head of the evolutionary venomics lab at the Indian Institute of Science and a lead author on the paper.
For one thing, snake venoms vary a lot between species, meaning treatment depends on which species has bitten you—which is not always known. Venoms can vary even within species; Sunagar’s research group discovered that an antivenom used after the bite of the monocellate cobra (Naja kaouthia) is almost completely ineffective in some regions in India.
Many antivenoms are made to work against several snakes from the same region, which means they’re relatively weak against one another. One such cocktail widely used in Africa fails to save roughly one in seven people bitten by the black mamba (Dendroaspis polylepis), for example. People have died despite receiving dozens of vials of the drug.
Also, because these drugs are made from animal proteins, they can cause adverse immune reactions, including life-threatening anaphylaxis. That’s why doctors often wait until a snakebite patient develops symptoms before administering antivenom, even though “any snake bite is a race against time: The quicker you get antivenom, the better the clinical outcome,” says Andreas Laustsen-Kiel, a toxinologist at the Technical University of Denmark who was not involved in the work.
To address these issues, Sunagar partnered with Casewell and Joseph Jardine, an expert in protein engineering at Scripps Research, as a part of a Wellcome Trust–funded consortium. Jardine’s team made lab-grown cells produce synthetic versions of a key ingredient of many snakes’ venom, known as long-chain three-finger alpha-neurotoxins (3FTx-L). These toxins cause paralysis by shutting down nerve cells’ ability to respond to a key neurotransmitter. Then the group tested some 100 billion artificial human antibodies in the lab’s massive antibody library—a far bigger collection than the immune system of any animal exposed to venom could come up with—to find out which one best bound to the toxins. “It’s really a needle in a very large haystack,” Jardine says. The researchers ended up with a couple dozen promising candidates.
These were sent to Casewell, whose team tested how well they protected human cells from the toxin. An antibody dubbed 95Mat5 had the best performance, and the team also found out why it worked so well: The antibody bound to alpha-bungarotoxin—the primary 3FTx-L in the venom of the Southeast Asian many-banded krait (Bungarus multicinctus)—at exactly same spot where the toxin binds ion channels on human nerve and muscle cells.
To find out whether 95Mat5 protects animals, Sunagar’s team injected groups of five mice with a normally lethal dose of alpha-bungarotoxin mixed with the antibody, a standard way to test antivenoms. All of the mice survived. 95Mat5 also saved mice injected with the full venom from the same krait species, believed to contain at least four dozen different toxins—even when given 20 minutes after the venom. The same was true for the venoms of monocellate cobras and black mambas. “If you had asked me 6 years ago, I would have said that you’d be out of your mind to think that you can neutralize a snake venom by targeting just one toxin,” Sunagar says.
The antibody did not protect mice from the venom of king cobras (Ophiophagus hannah)—a species found on the Indian subcontinent, in southern China, and in Southeast Asia—but it did delay their death.
Jardine says the team is planning to follow the same discovery process with other classes of potent snake toxins. Their distant hope is to create a cocktail of antibodies that neutralizes the venoms from every dangerous snake on the planet. “You’d no longer have to stock hundreds of antivenoms,” Jardine says. “You could stock a single universal one.”