Article by Lalita Panicker, Consulting Editor, Views and Editor, Insight, Hindustan Times, New Delhi
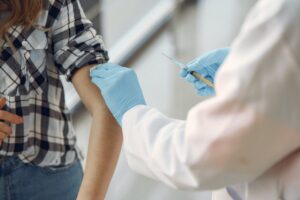
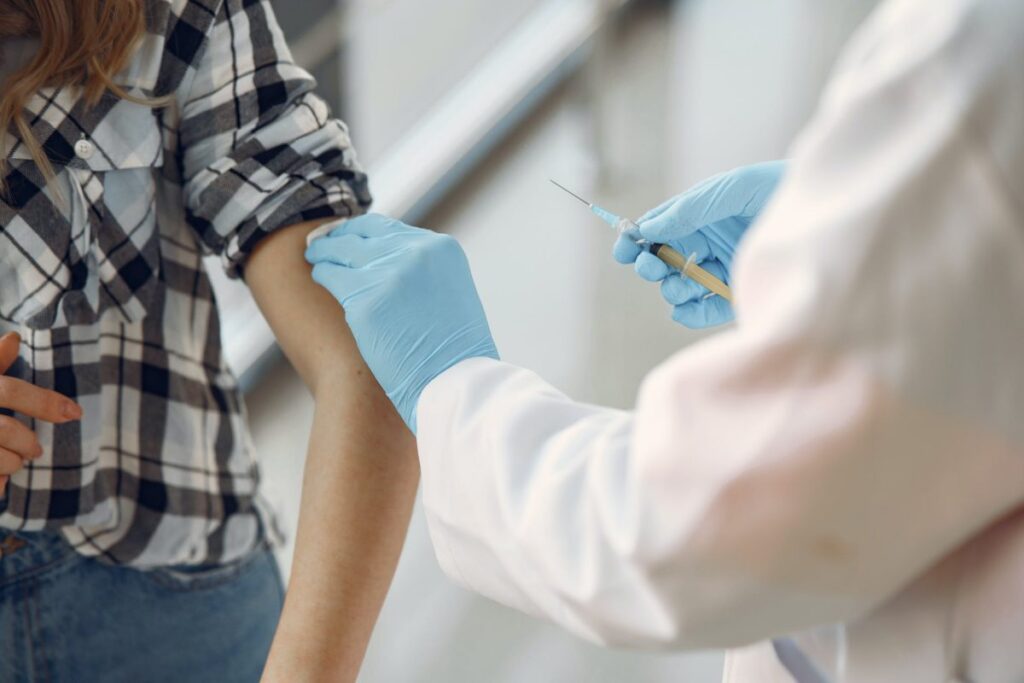
The world has run out of cholera vaccines—just when the deadly disease is on a rampage not seen in many years. Fifteen countries are currently reporting active outbreaks, with more than 40,900 cases and 775 deaths reported in January alone. But all available doses of oral cholera vaccines in the global stockpile have been allocated until mid-March, Philippe Barboza, cholera team lead at the World Health Organization (WHO), said on 23 February. He said there is now “no buffer for unforeseen outbreaks or preventive campaigns.” https://www.science.org/content/article/world-s-stockpile-cholera-vaccine-empty-relief-way?
The catastrophic shortage is a result not just of a surge in cases, but also of an overdependence on a single vaccine manufacturer, EuBiologics in Seoul, South Korea. But hope is on the horizon. EuBiologics is working to ramp up production of a simplified vaccine, and companies in South Africa and India are preparing to enter the market as well. The shortage “will lessen in 2024 and should be substantially addressed by 2025,” says Julia Lynch, director of the cholera program at the International Vaccine Institute (IVI), also in Seoul.
Many public health experts attribute the current surge in cholera, which began in late 2022, at least partially to climate change. Extreme weather events in Pakistan, Malawi, and Mozambique have destroyed health and sanitation infrastructure, allowing the bacterium to thrive. Armed conflict and displacement of people in the Democratic Republic of the Congo and Yemen have also led to outbreaks.
The two-dose oral cholera vaccine, developed by IVI based on earlier research in Vietnam, contains several strains of inactivated V. cholerae bacteria. Two doses given 2 weeks apart can offer robust protection for at least 3 years, and, when deployed early enough, can prevent outbreaks from ballooning.
The global stockpile of the vaccine, established in 2012, is managed by an International Coordinating Group on Vaccine Provision made up of experts from WHO, UNICEF, MSF, and the International Federation of Red Cross and Red Crescent Societies. It can rapidly send vaccine to countries in need. The number of doses available for shipment reached 36 million in 2023 and could be close to 50 million this year.
But all will be needed to fight ongoing outbreaks, and the shortage has forced some difficult choices. In late 2022, the coordinating group announced it would stop giving people second doses; even a single dose, studies have found, can provide substantial protection against cholera for a few years at least.
To boost supply, EuBiologics has simplified its original vaccine. The new formulation, Euvichol-S, contains two strains of the inactivated V. cholerae bacteria instead of five, and the recipe drops a heat inactivation step. That makes the vaccine easier to produce and about 20% cheaper. This will further expand EuBiologics production capacity by 38%, to about 52 million doses annually, according to a December 2023 press release from IVI. A phase 3 trial in Nepal in 2022 showed the simplified version protects as well as the original.
Euvichol-S is currently under review for “prequalification,” a seal of approval from WHO that the company expects to come through in April, says EuBiologics Director Rachel Park. Then Gavi, the Vaccine Alliance will procure the vaccine at roughly $1.5 per dose to replenish the stockpile. EuBiologics is also building a new facility that could expand its production capacity to 90 million doses annually.
To end the reliance on a single manufacturer, IVI in 2022 began to help Biovac, a South African company, set up a facility to produce the simplified vaccine. Biovac tells Science it plans to start clinical trials in 2025 and hopes to produce 30 million vaccine doses annually in 5 or 6 years. Biovac has been encouraged by a so-called advanced market commitment from Gavi, a pledge to buy many vaccine doses at established prices. So is the Indian manufacturer Biological E, which plans to produce IVI’s simplified vaccine.
Another Indian biotechnology company, Bharat Biotech, is working on its own low-cost cholera vaccine, Hillchol. Bharat has said little about its progress and did not respond to questions, but a phase 3 trial—at various locations in India—finished in early 2023, Lynch says.
////
Cecilie Knudsen placed the urine on one end of the strip, then sat anxiously for 15 minutes to see whether one or two lines appeared. Knudsen, a biotechnologist and co-founder of VenomAid Diagnostics, was waiting to see whether the test she and her colleagues developed would accurately detect the presence of a particular snake venom in a sample of mouse urine. It did. https://www.science.org/content/article/snake-just-bit-you-deadly-venom-pregnancy-test-could-tell?
The finding, published last month in Scientific Reports, represents “a really remarkable step in venom diagnosis,” says Kalana Maduwage, a physician and biochemist at the University of New England in Australia who has been working on similar diagnostic tools.
Snakebites kill some 100,000 people every year and disable hundreds of thousands more. The numbers could be reduced if doctors were able to administer antivenom more quickly and accurately. But only one test is available, and it doesn’t work in the regions where snakebites take the greatest toll.
Not every snakebite needs to be treated with antivenom. Most of the world’s snakes are not deadly, and even those that are don’t always inject venom when they bite. Administering antivenom in such cases means risking potentially severe side effects and wasting the rare, expensive drugs. But when a person does need treatment, speed is of the essence: With every second, venom toxins cause more damage, increasing the odds of disability or death.
In most parts of the world, the only way to tell whether antivenom is needed is to wait for symptoms to develop, Maduwage says. A diagnostic test could confirm that a person has venom in their body regardless of whether they’re showing symptoms—a signal to start treatment. “It’d prevent organ damage as well as the hospital stay,” Maduwage says.
Diagnostic tests could also tell doctors whether the venom was successfully neutralized by antivenom treatment: If subsequent tests are still positive, more antivenom is needed.
Not all antivenoms are the same. In places such as Brazil, doctors must choose the antivenom made for the species that bit. “If you get the wrong antivenom, not only could it have side effects, but it just won’t work,” says Selma Belfakir, a Ph.D. student at the Technical University of Denmark and a co–first author on the new Scientific Reports paper. But snakebite patients are often unsure what species bit them. So physicians try to tell from their symptoms, sometimes helped by a photo or a description of the snake—or its dead body.
That’s why the test developed by Belfakir, Knudsen, and their colleagues produces a band in one place if it’s a lancehead (Bothrops) and another if it’s a bushmaster (Lachesis)—the two genera responsible for the vast majority of deadly bites in the region. Bites by the two snakes require different antivenoms.
But there is no single toxin found in every snake’s venom. Every snake species has its own mix of dozens to hundreds of toxins. That makes it hard to develop a universal test, says Sakthi Vaiyapuri, a professor of pharmacology at the University of Reading. “We are not talking about just a single bacteria or virus here.”
Making regional tests for all of the most important snakes in one area is easier. In India, just four species are responsible for the vast majority of snakebites, Vaiyapuri notes, and all four are treated with the same antivenom. So he is working with the company ToxiVen Biotech, started by Harry Williams, one of his former Ph.D. students, to develop antibodies that detect all four venoms. A similar test would be invaluable in Africa, where the most widely used antivenom can be used for 11 of the region’s deadliest snakes.
A truly universal test is possible, Vaiyapuri says. Scientists just need to find the right antibodies. Just last week, an international team of researchers reported an antibody that binds to toxins found in snakes from Asia, Southeast Asia, and Africa.
Vaiyapuri is hopeful that his diagnostic test for Indian snakes will be available by the end of this year or early next. He plans to follow up with versions that identify the species as well and determine how much venom is present in the sample, which could guide antivenom dosing. Knudsen and colleagues say their test for Brazilian snakes is still years from the market.
////
Spanish researchers provided the first confirmation that a highly pathogenic strain of avian influenza has reached mainland Antarctica. Tests showed that two dead skuas found on 24 February near Argentina’s Primavera research station were infected with an H5 bird flu strain. A government press release didn’t say it was the H5N1 strain, but that seems likely. H5N1, previously found on islands in the Antarctic region, has killed millions of wild birds and poultry worldwide since 2021 and poses a threat to Antarctica’s dense penguin colonies.
////
Doctors and researchers at Tata Institute in Mumbai have developed a treatment, claiming that it could prevent cancer from occurring again. https://www.indiatoday.in/health/story/tata-institute-doctors-claim-new-drug-can-stop-cancer-resurgence-it-costs-rs-100-hundred-2508120-2024-02-28
This innovative tablet, which results from extensive research and testing over a decade, is believed to not only prevent cancer recurrence but also reduce side effects associated with treatments like radiation and chemotherapy by 50%. The results are from trials conducted on mice.
Dr Rajendra Badve, a senior cancer surgeon at Tata Memorial Hospital and part of the research group, explained the process behind the discovery.
“Human cancer cells were inserted in rats for the research, which formed a tumour in them. The rats were then treated with radiation therapy, chemotherapy and surgery. It was found that when these cancer cells die, they break into tiny pieces called chromatin particles. These particles can travel to other parts of the body through the bloodstream and when they enter healthy cells, they can turn them cancerous,” Dr Badve told NDTV.
In response to this problem, the researchers administered pro-oxidant tablets containing resveratrol and copper (R+Cu) to the rats. The R+Cu tablets generate oxygen radicals, effectively destroying chromatin particles.
When taken orally, these tablets release oxygen radicals in the stomach, quickly entering the bloodstream. This process prevents the release of cell-free chromatin particles in circulation and inhibits the movement of cancer cells, a process called metastases.
The researchers also claim that R+Cu tablets mitigate the toxicity associated with chemotherapy.
This discovery, referred to as the “Magic of R+Cu,” is expected to reduce the side effects of cancer treatment therapy by approximately 50% and demonstrate 30% efficacy in preventing cancer recurrence.
The tablet is anticipated to be effective against cancers affecting the pancreas, lungs, and oral regions.
The doctors are currently awaiting the approval of the Food Safety and Standards Authority of India (FSSAI). Once approved, the tablet is expected to be available in the market by June-July.
Dr Badve added that “compared to the millions spent on the development, the tablet could cost less than Rs 100.”
While the tablet’s impact on side effects has been tested on both rats and humans, prevention tests have been conducted only on rats.
Human trials are expected to take not less than five years to complete.