Author CDC/ Cynthia S. Goldsmith, Inger K. Damon, and Sherif R. Zaki
A clinical trial to evaluate the antiviral drug tecovirimat, also known as TPOXX, in adults and children with monkeypox has begun in the Democratic Republic of the Congo (DRC). The trial will evaluate the drug’s safety and its ability to mitigate monkeypox symptoms and prevent serious outcomes, including death. www.nih.gov/news-events/news-releases/monkeypox-treatment-trial-begins-democratic-republic-congo?
The National Institute of Allergy and Infectious Diseases (NIAID), part of the US National Institutes of Health, and the DRC’s National Institute for Biomedical Research (INRB) are co-leading the trial as part of the government-to-government PALM partnership. Collaborating institutions include the US Centers for Disease Control and Prevention (CDC), the Institute of Tropical Medicine Antwerp, the aid organization Alliance for International Medical Action (ALIMA) and the WHO.
TPOXX, made by the pharmaceutical company SIGA Technologies, Inc. (New York), is approved by the US Food and Drug Administration for the treatment of smallpox. The drug impedes the spread of virus in the body by preventing virus particles from exiting human cells. The drug targets a protein that is found on both the virus that causes smallpox and the monkeypox virus.
“Monkeypox has caused a high burden of disease and death in children and adults in the Democratic Republic of the Congo, and improved treatment options are urgently needed,” said NIAID Director Anthony S. Fauci, M.D. “This clinical trial will yield critical information about the safety and efficacy of tecovirimat for monkeypox. I want to thank our DRC scientific partners as well as the Congolese people for their continued collaboration in advancing this important clinical research.”
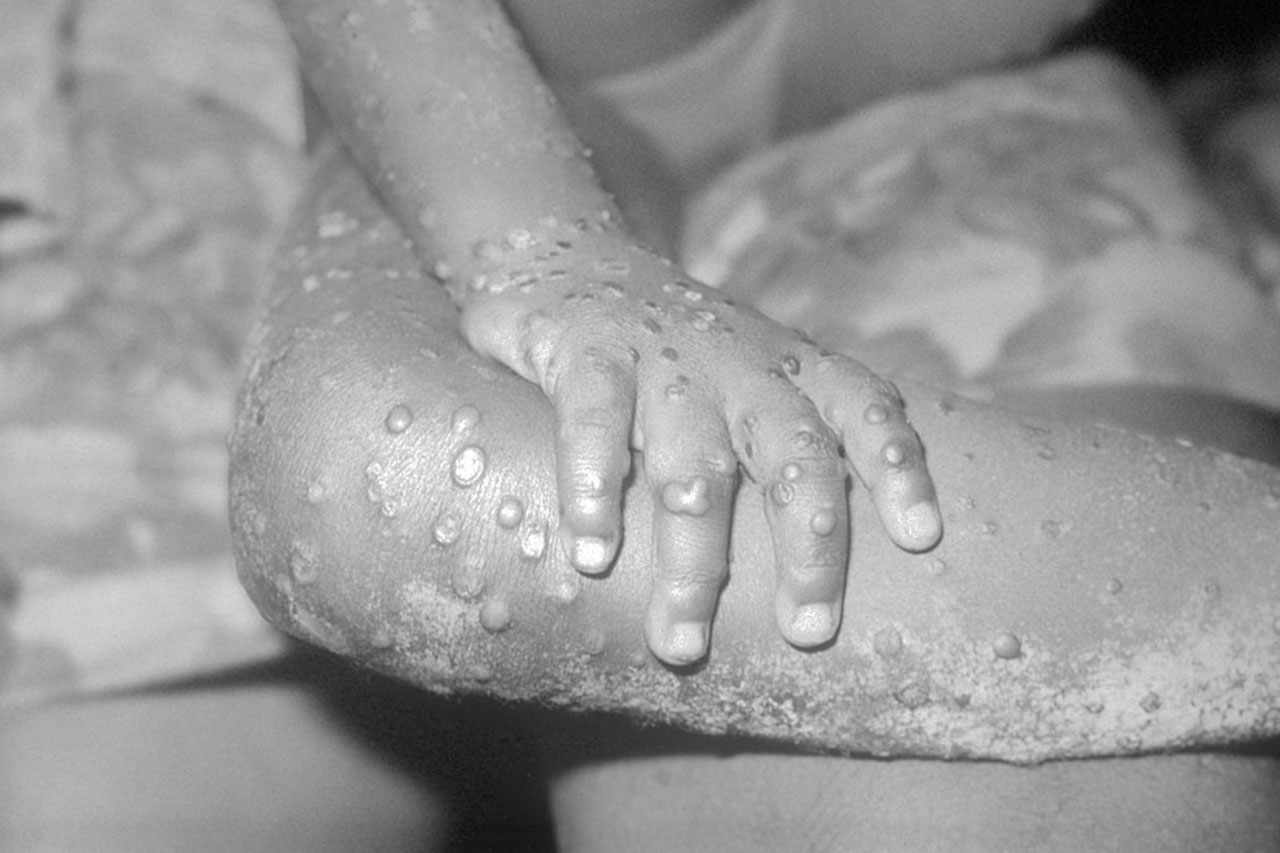
From January 1, 2022 to October 5, 2022, the WHO has reported 68,900 confirmed cases and 25 deaths from 106 countries, areas and territories.