The U.S. Food and Drug Administration last week approved a genetic treatment for the blood disorder beta-thalassemia, marking the third U.S. gene therapy for a rare disease. www.science.org/content/article/news-glance-new-gene-therapy-europe-s-drought-and-black-hole-s-photon-ring?
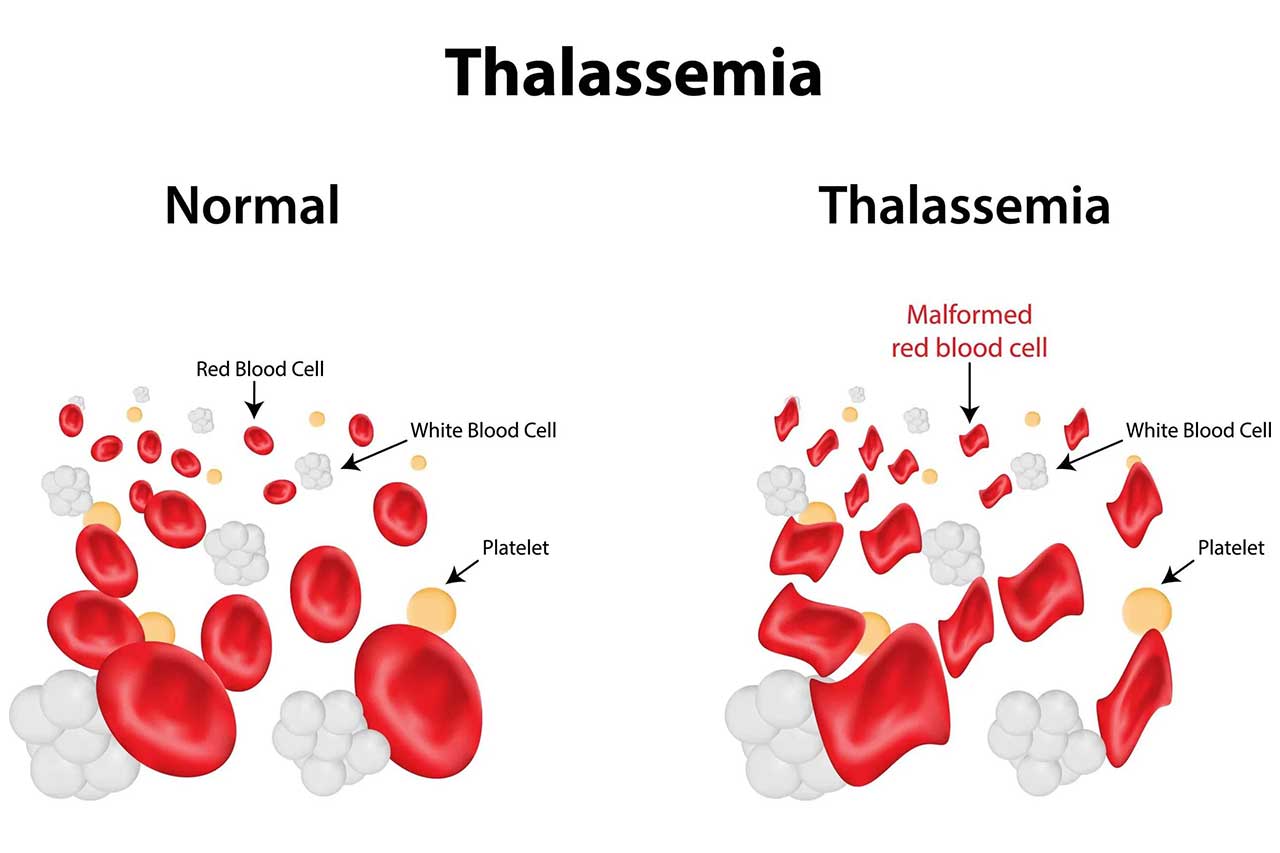
The disorder causes low haemoglobin and severe anaemia, and the regular blood transfusions used to treat it can cause iron build-up that damages organs. The new treatment, Zynteglo, from manufacturer Bluebird Bio, relies on a virus to deliver a gene for haemoglobin into the patient’s bone marrow cells, grown in culture; the cells are then infused back into the body. In clinical trials, 89% of treated patients no longer required transfusions. Zynteglo won European approval in 2019 but was removed from the market after countries baulked over the high price; in the United States, it will cost $2.8 million per one-time treatment, making it one of the most expensive drugs ever. Bluebird is testing a different product that uses the same method for sickle cell anaemia, which is more common in the United States than thalassemia.