Article by Lalita Panicker, Consulting Editor, Views and Editor, Insight, Hindustan Times, New Delhi
Gavi, the Vaccine Alliance, has committed up to $1 billion to bolster Africa’s ability to sustainably produce its own doses of lifesaving vaccines. Manufacturers based in Africa produce only 1% of the vaccine doses used on the continent. https://www.science.org/content/article/news-glance-ai-rules-europe-vaccines-africa-and-union-nih-early-career-researchers?
Last week, Gavi announced that with money left over from the COVID-19 Vaccines Global Access Facility—an effort to provide an equitable distribution of COVID-19 vaccines—it would create the African Vaccine Manufacturing Accelerator (AVMA) to focus on preventing 11 priority infectious diseases. As an incentive to begin making the doses, AVMA will give a manufacturer $10 million to $25 million for each vaccine that the World Health Organization determines meets international quality standards. If Gavi and its partner, UNICEF, agree to buy the vaccine, AVMA will “top up” the price paid by as much as 50 cents extra per dose as an “accelerator payment.” Its goal is to help at least four African manufacturers supply a total of more than 800 million doses over a decade.
////
If your immune system or drugs can’t stop a viral infection, why not pit a virus against itself? That’s the provocative idea several labs are pursuing. They are studying whether deliberately introducing engineered viruses into people infected with their natural relatives can “drive” a foreign gene into those viruses that ultimately wipes out an infection. https://www.science.org/content/article/fighting-viruses-viruses-gene-drive-offers-new-strategy-beat-infections?
No lab has knocked down an infection in animals this way yet, but a group has now shown it’s theoretically possible. These so-called gene drives harness the genome editor CRISPR to perform genetic surgery that speeds the spread of a gene through progeny. So far, scientists have received the most attention for adding gene drives to animals such as rodents and mosquitoes to control their numbers. But in a preprint last week, the team reported a similar feat with herpesvirus-1 (HSV-1). When both engineered and unmodified herpesviruses were inoculated into mice, the gene drive converted up to 90% of the viruses—possibly enough to prevent an HSV-1 infection from causing symptoms such as painful cold sores. A second group has succeeded in putting gene drives into HSV-1 that is growing in infected cells in lab dishes.
The viral gene drive work is a long way from curing an infected person. No one knows, for example, what kind of genetic modification the drive should propagate to drive down an infection. But other scientists see its potential.
Researchers have developed gene drives mostly in animals that sexually reproduce. They’ve created genetically modified females or males whose odds of spreading a gene to their offspring are significantly higher than 50%—the typical Mendelian chance that a descendant will inherit a particular gene variant. Most experiments aim to spread modifications that kill the offspring or render them sterile, a possible strategy for pest control. Such work has only been done in labs so far, however, because of concerns that releasing animals with gene drives could have dire consequences, such as accidentally wiping out an entire species or harming animals that aren’t the target.
Unlike people, viruses don’t have gene-scrambling sex with each other when they replicate. They simply command infected cells to read their genes and produce new viruses. But if multiple virions infect a single cell—as happens with herpesviruses—they often do something akin to sex, randomly swapping genetic sequences inside the nucleus. This “recombination” leads to viral progeny that spread the new genomes, and a gene drive hijacks this natural process to introduce and boost genetic changes that might ultimately disable a whole population of viruses.
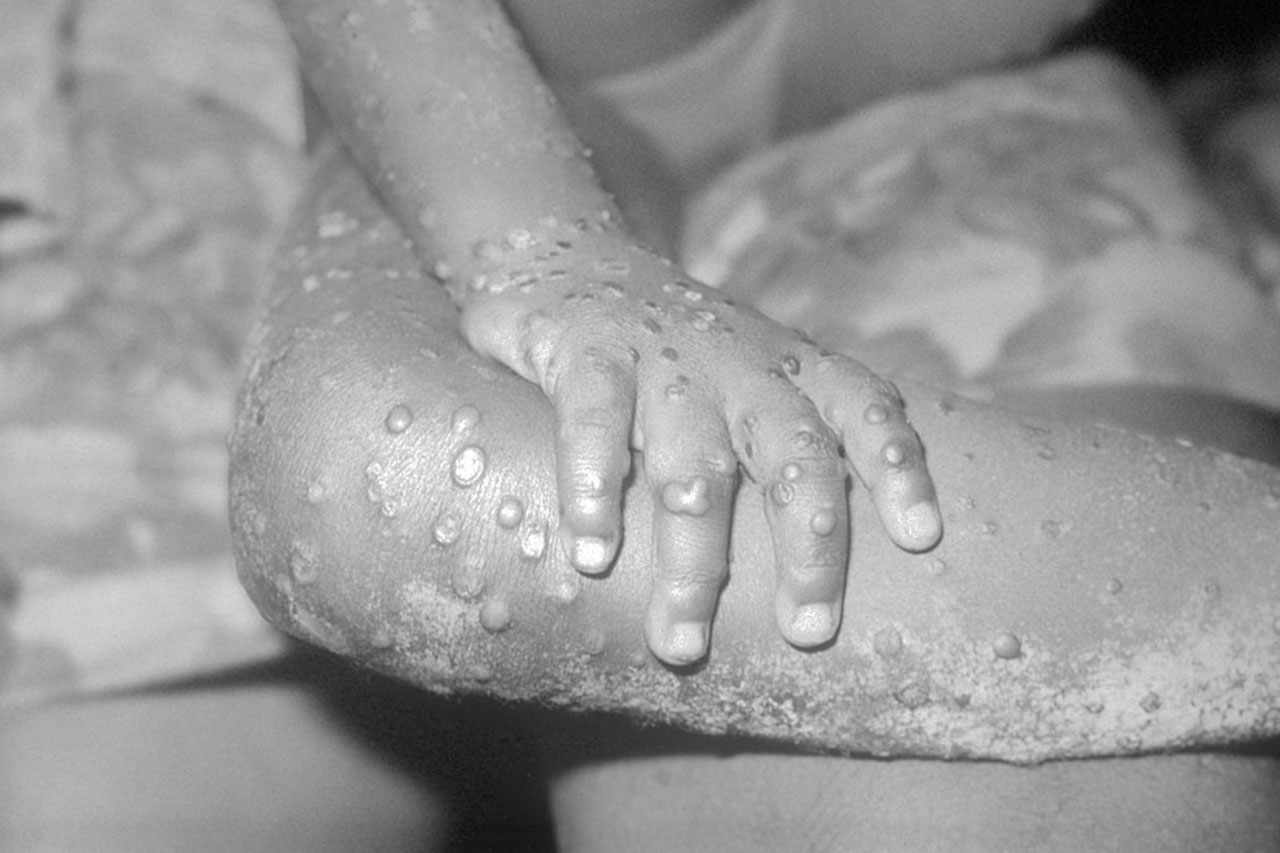
HSV-1, HSV-2, human cytomegalovirus (hCMV), Epstein-Barr virus, and other herpesviruses are particularly attractive candidates for tackling with a gene drive because they can cause lifelong, latent infections that periodically flare and cause symptomatic disease. Some drugs can tamp down viral reactivation, but in people who have compromised immune systems, those drugs often fail and herpesviruses run riot, damaging many parts of the body and even causing death. This is a particular problem with people who have untreated HIV infections or receive organ or bone marrow transplants.
////
French President Emmanuel Macron last week established the country’s first multidisciplinary scientific advisory council to help him “fully put science at the heart” of political decisions. (Other, specialized panels have advised the government on climate change and the pandemic.) Comprising 12 French scientists including two Nobel laureates and five women, the council will meet privately with Macron each quarter; its remit includes proposing new projects to build. Unlike some other countries, France does not have the position of chief scientific adviser. https://www.science.org/content/article/news-glance-ai-rules-europe-vaccines-africa-and-union-nih-early-career-researchers?
////
In what has already been tagged as a “game-changer” for cancer treatment, the potent once-a-day tablet known as divarasib has continued to impress at Phase 1b trial stage, outperforming not just current therapies but its previous trial results. https://newatlas.com/medical/pill-bowel-cancer/?
Following on from a standalone clinical trial earlier this year, divarasib has now been combined with the existing targeted therapy drug cetuximab, and has delivered a 62.5% positive outcome for people with advanced or metastatic colorectal cancer (CRC) linked to the KRAS G12C gene mutation.
In the first trial, at the Peter MacCallum Cancer Centre in Australia, CRC patients receiving just divarasib had a 35.9% positive response rate, which was considered extremely promising.
KRAS is a key protein that regulates how cancer cells behave. For cancer patients with the KRAS G12C gene mutation, their cancer cells are far more likely to divide uncontrollably and form tumours, making the disease very challenging to treat with existing medication. As such, even though it affects around 4% of CRC patients, it has a poor prognosis.
The latest research, again led by Professor Jayesh Desai at the Peter MacCallum Cancer Centre, indicates that when taken in conjunction with cetuximab, divarasib targets this mutation and is effective at slowing the development of tumours, while also being well tolerated with few adverse effects.
“The median progression-free survival for patients in the study was just over eight months and the treatment was well tolerated with manageable side effects,” Desai said. “While this is not a head-to-head trial, the response rates are better than what we have seen with other treatments that work on the KRAS G12C mutation pathway.
“We are very hopeful that this combination of divarasib with cetuximab will translate into better outcomes for our colorectal cancer patients,” he added.
While the KRAS G12C mutation is perhaps most associated with CRC, it plays a key role in the expedited progress of other cancers, such as non-small cell lung cancer (detected in around 13% of patients).
Current treatment for KRAS G12C-positive CRC patients includes 5-FU-based chemotherapy with irinotecan, oxaliplatin and/or capecitabine, however, it faces limitations due to low targeting of specific tumours and toxicity.
Earlier this year, the cancer centre began a global Phase I trial of divarasib treatment on 137 cancer patients. Research found that the drug was 50 times more specific and 20 times more potent than other similar agents that are currently being used to treat the KRAS mutation.
////
Nature Medicine asked specialists to name the 2024 clinical trial that they were most excited about. These were some of the responses:
- A trial of around 150,000 people at six hospitals in the United Kingdom will test whether artificial intelligence can analyse computed tomography scans to catch lung cancer earlier. The hope is that AI will cut the time to diagnosis by up to 50%.
- The NADINA trial will compare the efficacy of the immunotherapies ipilimumab and nivolumab when given before the main cancer treatment with that of nivolumab administration after the main treatment in 420 people with stage 3 melanoma. This trial could be practice-changing if the treatment improves survival.
- DESTINY-Breast12 will test an antibody–drug conjugate in people with advanced, HER2-positive breast cancer. Some of the people in the trial will have cancer that has spread to their brain, and some will not. This could help researchers to determine whether this drug can cross the blood–brain barrier.
- The 4-IN THE LUNG RUN trial will test whether reducing the frequency of lung-cancer screening from once per year to once every two years will affect deaths across 26,000 people in Europe.