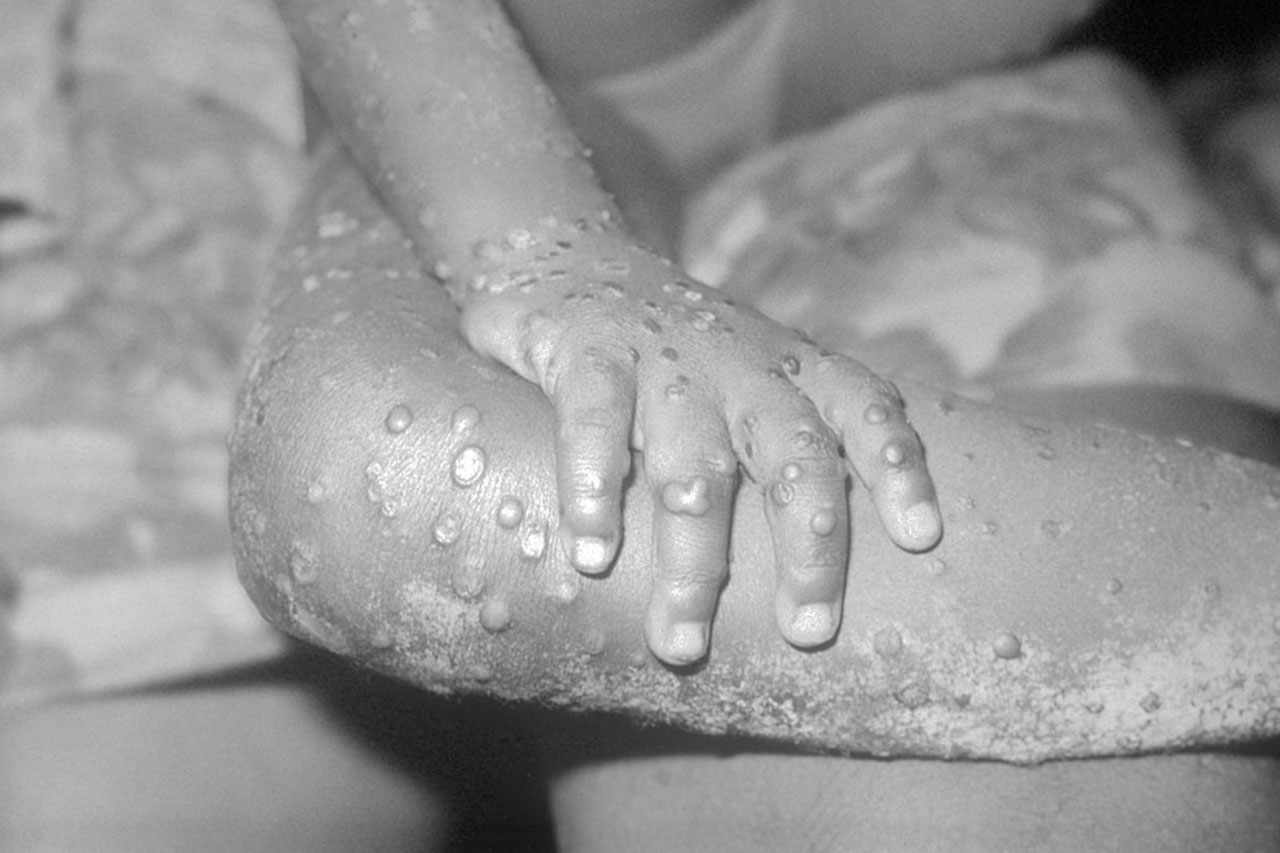
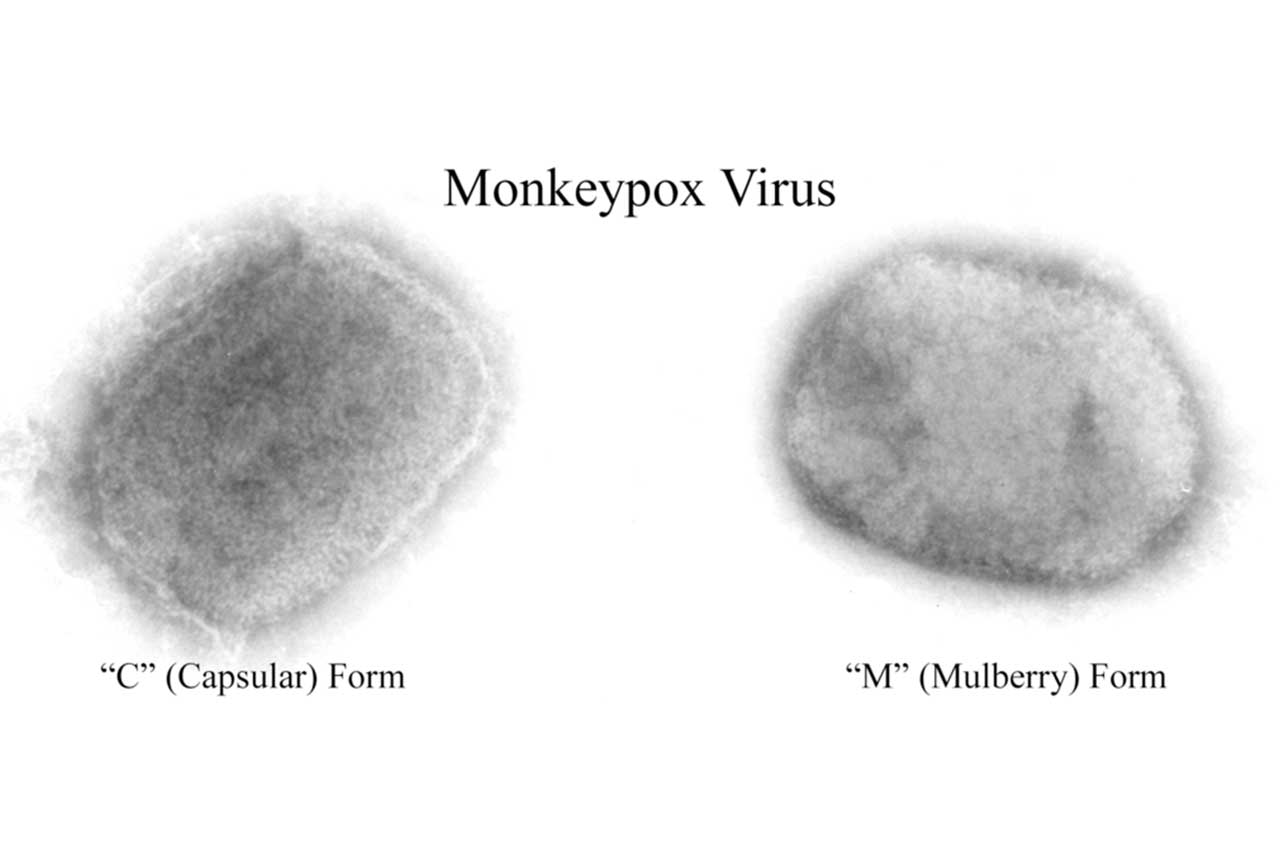
Article by Lalita Panicker, Consulting Editor, Views and Editor, Insight, Hindustan Times, New Delhi
At last, the world’s largest biomedical research agency has a permanent leader. The U.S. Senate recently voted 62-36 to confirm oncologist to direct the U.S. National Institutes of Health (NIH). https://www.science.org/content/article/u-s-senate-confirms-monica-bertagnolli-nih-director?
The confirmation of Bertagnolli, who is expected to be sworn in within days replacing acting Director Lawrence Tabak, brings relief to the biomedical research community. Researchers had been waiting for a new NIH chief for a long time ever since geneticist Francis Collins stepped down as Director in December 2021. President Joe Biden nominated Bertagnolli in May.
A cancer surgeon and clinical trial expert, Bertagnolli will be NIH’s 17th director and the second woman to lead the agency. She left Harvard Medical School and affiliated hospitals to head the National Cancer Institute in October 2022. She now takes over the reins of the $47 billion NIH as it faces enormous budget and political pressure and with just 15 months remaining until the end of Biden’s term—and perhaps her tenure. “She will have plenty of problems on her plate,” says cancer researcher Harold Varmus, who headed NIH in the 1990s.
For example, the substantial number of Senate votes against Bertagnolli reflects concerns among both conservative and liberal lawmakers about NIH policies.
Conservative lawmakers have complained about NIH’s role in funding politically sensitive research such as gender-affirming care and virus studies that some claim led to the COVID-19 pandemic. Two liberal senators, Bernie Sanders (I–VT) and John Fetterman (D–PA), voted against her confirmation because of concerns that NIH is not doing enough to bring down drug prices.
At her confirmation hearing before the Senate health committee last month, Bertagnolli said she wants to bring NIH’s innovations and clinical trials to diverse populations in the United States. One immediate challenge she will face is urging Congress to preserve NIH funding as it works to reconcile a 2024 spending measure passed by the Senate that would slightly increase the agency’s budget with a bill from the House of Representatives calling for deep cuts.
The research community hopes Bertagnoli will also address other matters, including reversing NIH’s shift away from basic research toward more applied studies. Also awaiting Bertagnolli is an advisory panel’s report, due in December, on low salaries for postdocs and the increasing difficulty senior scientists face in recruiting that pivotal workforce. She is also expected to be pressed to clarify NIH’s relationship with the Advanced Research Projects Agency for Health, a new independent agency within NIH that Biden created to boost high-risk, high-impact research.
It’s a lot to pack into a tenure that might not last beyond Biden’s term if he fails to win re-election.
/////
The antifungal Amphotericin B (AmB) is an old and effective drug—it saved many COVID-19 patients whose compromised immune systems failed to stop secondary fungal infections. But it sometimes causes life-threatening kidney damage. Now, after more than a decade of sleuthing into this toxicity, researchers have not only found an explanation, but used it to devise a powerful antifungal alternative without any obvious side effects in mice and human cells.
“This is really inspiring work,” says Leah Cowen, a mycologist at the University of Toronto. “They leveraged molecular insights into how the drug works to dial up the properties they wanted and dial down properties they didn’t want.” https://www.science.org/content/article/new-antifungal-kills-without-toxic-side-effects?
Worldwide, fungal diseases kill some 1.5 million people annually, about the same as tuberculosis or malaria. But in contrast with antibiotics, where dozens of classes of effective drugs are available, there are only three classes of antifungals, and each faces problems of toxicity, growing resistance, or limited effectiveness.
AmB, produced by a Streptomyces bacterium, was first isolated in 1955 from soils near the Orinoco River in Venezuela. And though chemists learned to synthesize it, the complex molecule was painstaking to make from scratch. In 2012, researchers led by Martin Burke, a chemist at the University of Illinois Urbana-Champaign (UIUC), reported they had developed a way to quickly produce closely related analogs of AmB from modular building blocks. And tests on one such analog revealed that AmB kills fungi by stripping them of ergosterol, a key structural support in their cell membranes. Human cells don’t use ergosterol. But cholesterol, a closely related sterol, performs much the same function in human cells. And Burke and his colleagues found that AmB likely causes renal damage by stripping cholesterol out of the membranes of kidney cells and weakening them.
Burke’s team churned out numerous new variants using various techniques, each with a slight tweak to its chemical structure, to see whether any had reduced toxicity. One was initially promising enough to license to a biotech company, but ultimately proved not safe enough in animal studies.
Burke and his colleagues went back to the drawing board. They developed ideas on where tweaks to the molecule might further reduce its toxicity by studying high-resolution images of AmB binding to both ergosterol and cholesterol recently provided by Chad Rienstra’s group at the University of Wisconsin-Madison.
Last week in Nature, Burke and his colleagues reported that in cell culture the compound, dubbed Am-2-19, is at least as effective as AmB, if not more so, in killing more than 500 different fungal species. Studies on mice showed that Am-2-19 thwarted three common, hard-to-treat fungal infections with no signs of toxicity, even at high doses. And tests on human blood and kidney cells produced no warning signals. Am-2-19 has been licensed to Sfunga Therapeutics, which has launched a phase 1 human safety trial.
////
The drugs aren’t working as well as they used to.
That’s the sobering takeaway from new research published in The Lancet Regional Health – Southeast Asia last week: The most commonly prescribed antibiotics in Southeast Asia are now only 50% effective at treating sepsis and meningitis in new-borns.
And that’s a serious setback. Sepsis kills 1 in 5 patients. Meningitis is responsible for a quarter of million deaths a year – half among children under the age of 5. Overall, childhood infections are responsible for over 550,000 deaths each year.
Why aren’t the drugs doing their job? It’s because overuse of those drugs has led to the evolution of antimicrobial resistant infections – bacteria and other diseases that are no longer knocked out by treatment. https://www.npr.org/sections/goatsandsoda/2023/11/07/1209109088/antibiotics-that-fight-deadly-infections-in-babies-are-losing-their-power?
Dr Phoebe Williams, a physician and lecturer at the University of Sydney, Australia School of Medicine and lead author of the new research, says that these antimicrobial resistant infections are responsible for “around 5 million deaths each year” of both children and adults.
Roughly 1 million of those deaths each year occur in Southeast Asia.
Babies are especially vulnerable. While an adult’s immune system is often strong enough to fight off these infections, children who haven’t had the chance to build up their immunity suffer the brunt of the consequences.
In the last 30 years, better health care for mothers and infants has helped cut child mortality in half, according to the World Health Organization; deaths have dropped from 5 million per year in 1990 to 2.4 million in 2020. But infections still pose one of the greatest threats to new-borns. And the rise of these antimicrobial resistant infections is a major obstacle for the U.N.’s goal of ending all preventable neonatal deaths by 2030.
Williams’ research shows that in Southeast Asia these infections are increasingly antimicrobial resistant. “One of the units we work with in the Philippines, for example, their mortality rates in neonatal sepsis have gone from being about 20% ten years ago to 75% in the last 2 years,” Williams says.
Among the research findings is the discovery that many of the drugs recommended in the 2013 World Health Organization guidelines for treating childhood infections no longer work, according to Ramanan Laxminarayan, a senior research scholar at Princeton University who wasn’t involved in the new research.
Ceftriaxone, one of the recommended drugs, is now only effective at treating 1 in 3 neonatal meningitis cases. Another recommended drug, gentamicin, only cures neonatal sepsis and meningitis half the time.
Laxminarayan says that the WHO guidelines are being revised to recommend more effective treatments. In the meantime, many clinicians in the affected countries are left to figure out the most appropriate course of action themselves.
Williams says that the solution is to take a closer look at both old and new medicines to identify the most effective treatments for children.
She says that using older drugs “is probably one of our strongest bets because those agents are off patent.” So cheaper generic versions are available now that the original patents have expired. And because these medications haven’t been widely used for years, some infections may no longer be resistant to them.
Newer drugs that can fight off these antimicrobial resistant infections have been developed but rarely get approved for use in children. Williams says that “since the year 2000, 40 antibiotics have been licensed for adults and 4 in babies, despite babies having a far bigger burden of AMR.” Williams says that the reason many of these drugs don’t get approved for children is because they require different dosing regimens than adults. Figuring out the correct dose for children is often not a priority when companies first seek approval.
One class of drug that Williams’ research has shown to still be effective and that is approved for children are carbapenems, a collection of powerful antibiotics containing many different drugs that are given by IV and often used as a last resort for treating bad infections. Carbapenems typically aren’t prescribed unless absolutely necessary because they’re expensive, the IV is an invasive element and they’re not widely available in every country.
“They’re not in the WHO guidelines,” Williams says, but “we know many clinicians globally are now going to them as a first-line agent.”
Carbapenems may currently be the most effective antibiotic available, but resistance is growing to them too. Using this ‘last resort’ drug as a first line of defence in the long run will make the antimicrobial resistance problem worse.
Even now the carbapenems don’t always work. “This particular study shows only 81% coverage [from carbapenems] which means that 19% of the time those drugs are not going to work,” Laxminarayan says. “I don’t know about you, but I wouldn’t want to have a child with an infection where 19% of the time the most effective drug that I have doesn’t work and the child is at risk of dying.”
Both Laxminarayan and Williams stress that the paper’s findings are not confined to southeast Asia. “There’s tens of thousands of carbapenem-resistant infections in the U.S. each year,” Williams says.
It’s a problem Laxminarayan thinks should have been addressed a long time ago. “Unfortunately, the world only responds to crises and now we’re seeing a crisis with new-born survival,” he says.