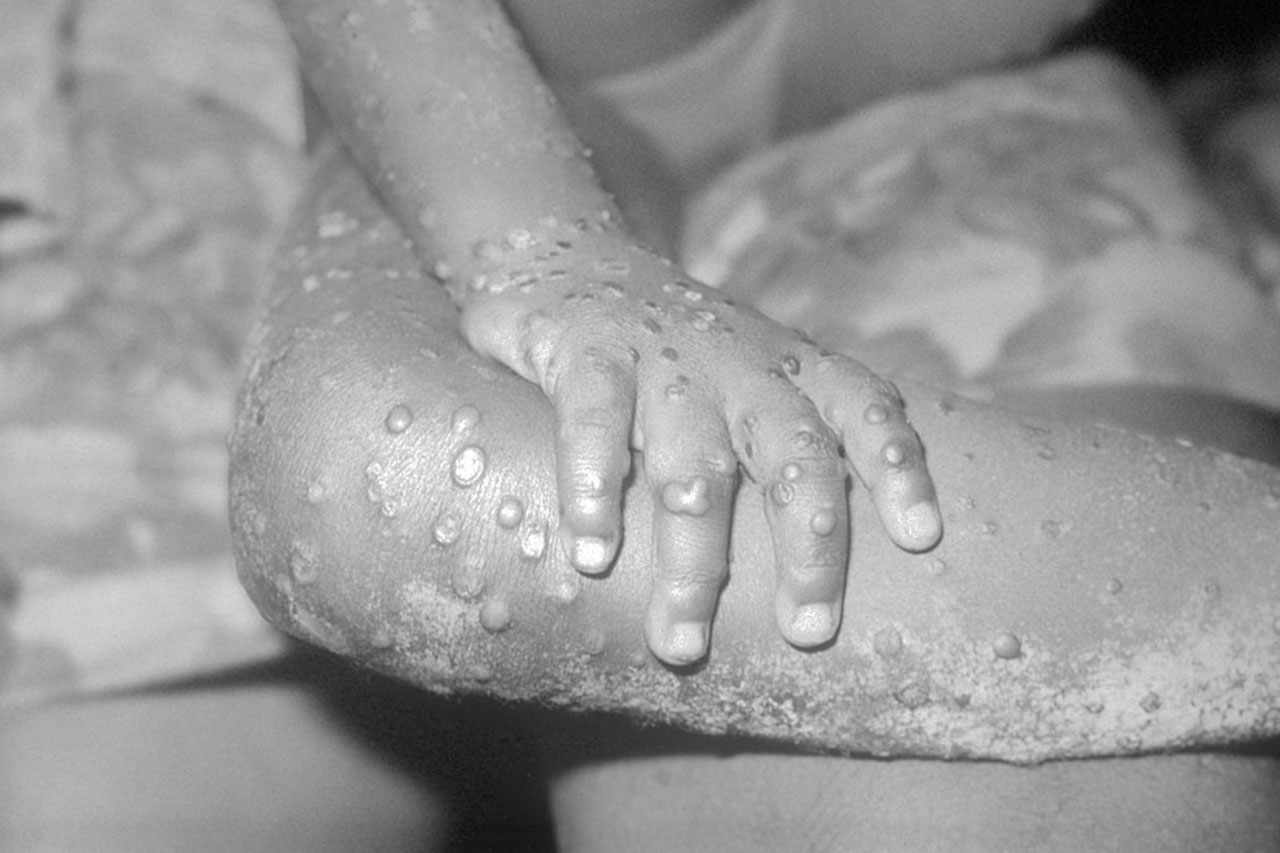
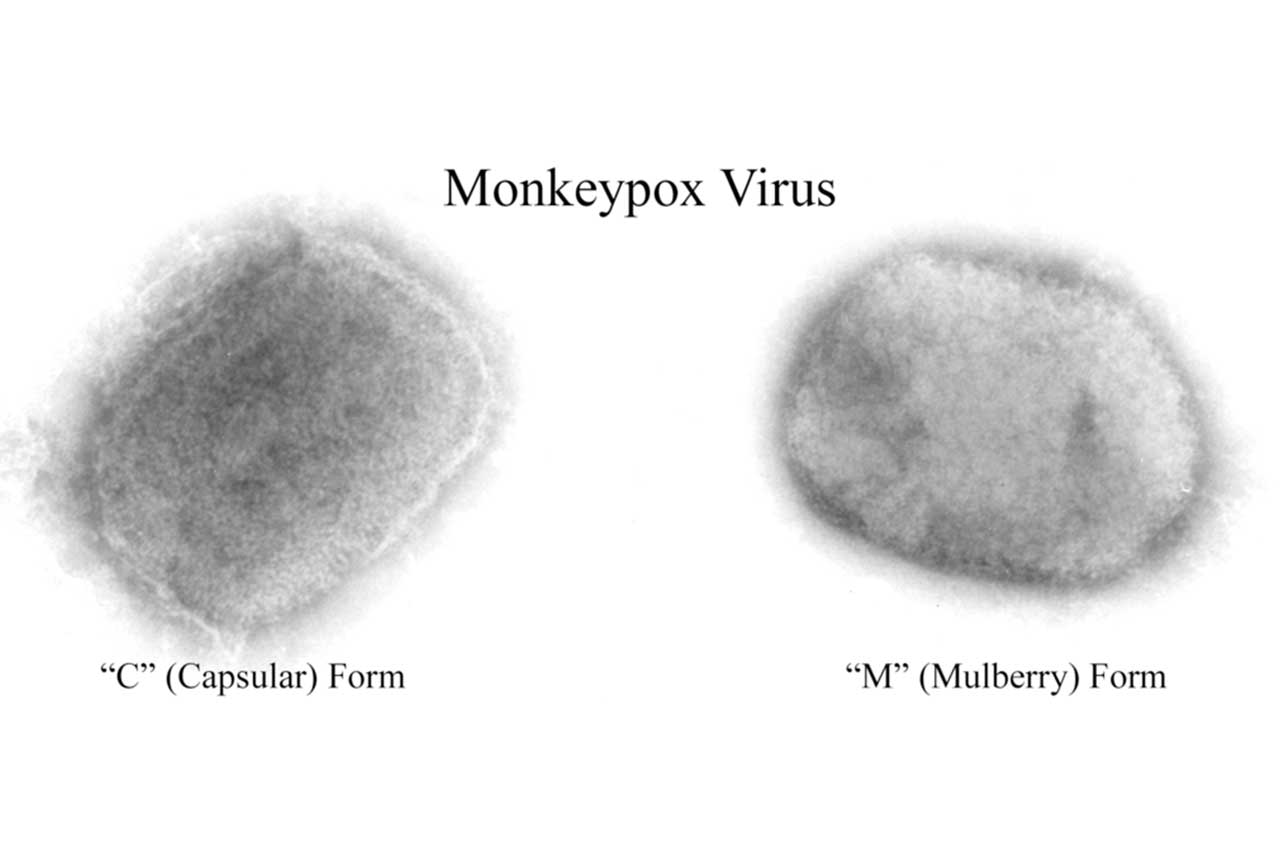
Article by Lalita Panicker, Consulting Editor, Views and Editor, Insight, Hindustan Times, New Delhi
DNA-based screening methods for high-risk human papillomavirus (HPV) have proven to be highly effective for early cervical cancer detection but remain too expensive and difficult to use in resource-limited settings. Kundrod et al. have developed a prototype point-of-care test for detecting HPV16 and HPV18.
https://www.science.org/doi/full/10.1126/science.add3434?et_rid=785235729&utm_campaign=SCIeToc&af=R&et_cid=4784075&utm_medium=email&utm_content=alert&utm_source=sfmc
This test uses isothermal DNA amplification and lateral flow detection with low-cost components that can detect HPV DNA at a clinically relevant limit of detection. This test was successfully demonstrated in a high-resource setting in the United States and in a low-resource setting in Mozambique, and it could be performed by minimally trained personnel at an estimated cost of less than $5 per test.
////
Orforglipron, Eli Lilly’s investigational oral daily nonpeptide glucagon-like peptide-1 (GLP-1) agonist that can be taken with or without food, appears comparable with other injectable and oral agents in the class for treating obesity and type 2 diabetes, suggest two new phase 2 studies. https://www.medscape.com/viewarticle/993667?ecd=wnl_edit_tpal&uac=398271FG&impID=5567738&faf=1
Currently approved GLP-1 agonists for type 2 diabetes and/or obesity are peptide-based and given by subcutaneous injection or orally. No oral GLP-1 agonists are currently approved for obesity, and only one, oral semaglutide (Rybelsus, Novo Nordisk), is approved for type 2 diabetes.
Oral semaglutide is formulated with an ingredient that helps protect it from degradation and enhances gastric absorption. To maximize absorption and efficacy, patients are advised to take it in the fasting state and not to eat or drink anything for at least 30 minutes after. Nevertheless, the bioavailability of orally ingested semaglutide is only 1% or less.
Orforglipron, in contrast, is a small molecule that isn’t a peptide, so it isn’t degraded in the gastrointestinal tract, Juan Frias, MD, lead author of the phase 2 trial in type 2 diabetes, told Medscape Medical News.
“It’s a chemical, a small molecule that also acts on the GLP-1 receptor, but because it’s not a protein it’s not degraded by enzymes. It’s like any other pill. Earlier pharmacokinetic studies have shown that taking it with or without food doesn’t make a difference,” said Frias, who is principal investigator at Velocity Clinical Research, Westlake, California.
The results of the phase 2 trial of orforglipron in the treatment of adults with overweight or obesity were presented here June 23 at the American Diabetes Association (ADA) 83rd Scientific Sessions, and the findings were simultaneously published in the New England Journal of Medicine.
The type 2 diabetes data were also presented, as a poster, at the ADA meeting, and simultaneously published in The Lancet.
Asked to comment, moderator of the session during which the obesity study was presented, Elisabetta Patorno, MD, DrPH, told Medscape Medical News: “I think the findings are exciting. It is a formulation that patients might be able to tolerate much more, in terms of not having these very strict restrictions on how to take [oral semaglutide]. It’s very cumbersome. They have to be fasting. They have to wait to eat.”
Of course, orforglipron must go through much further testing, noted Patorno, associate professor of medicine at Harvard Medical School, Boston, Massachusetts.
///
A new US start-up, STAT Health, has emerged from the shadows with $5.1 million in funding to develop an in-ear wearable device that can measure blood flow in the brain, which it says is the “missing vital sign”.
https://pharmaphorum.com/news/ear-wearable-start-stat-has-long-covid-its-sights?
According to the company, the earbud-like device could help researchers get to the bottom of complex and poorly understood health conditions like long COVID.
What these disorders have in common are symptoms such as dizziness, brain fog, headaches, fainting, and fatigue, that can get worse when the patient stands up and are all thought to be associated with reduced cerebral blood flow (CBF).
That can be measured using ultrasound technology, but it’s a complex, expensive and cumbersome procedure, and has to be carried out in a clinic. As a result, physicians often use using heart rate and blood pressure data to infer what might be happening in the cerebral circulation, which lacks accuracy.
In contrast, STAT Health’s device uses an optical sensor and artificial intelligence to gauge cerebral blood flow by using flow in a shallow ear artery as a proxy. It also includes an accelerometer to detect when a patient stands up, as well as pressure and temperature sensors to provide other data, all delivered 24 hours a day, seven days a week if needed.
STAT Health’s device has been put through its paces by researchers at Johns Hopkins University in the US, who published a study in March showing that the wearable can detect fainting minutes before it happens.
////
The World Health Organization’s probe into contaminated cough syrups, which have been linked to nearly 300 worldwide deaths so far, has flagged 20 such toxic medicines originating from two countries — India and Indonesia. https://indianexpress.com/article/india/toxic-syrups-who-probe-flags-20-products-in-india-indonesia-8673447/
In response to an email by The Indian Express, WHO spokesperson Christian Lindmeier said these 20 products were manufactured by “15 different manufacturers” in the two countries.
All the medicines are syrups — cough medicine, paracetamol or vitamins. These would include the 15 previously identified contaminated syrups, seven of which were manufactured in India by Haryana-based Maiden Pharmaceuticals (4), Noida-based Marion Biotech (2), and Punjab-based QP Pharmachem (1). The rest were made in Indonesia.
The WHO has already raised ‘medical product alerts’ on the 15 medicines in Gambia and Uzbekistan, where Indian-made syrups were linked to at least 88 deaths last year, as well as in Micronesia and the Marshall Islands. It also raised an alert in Indonesia, where the syrups, sold domestically, were linked to the death of more than 200 children.
Earlier this June, the Nigerian drug controller raised an alert after it found a paracetamol syrup sold in Liberia contaminated with diethylene glycol or ethylene glycol. The syrup was manufactured by a Mumbai-based company.
Medical product alerts are raised to ensure that more people do not consume the contaminated medicine and the products are removed from the supply chain. Lindmeier said WHO raises such alerts only when there is sufficient evidence to demonstrate that a product is contaminated.
On being asked whether the instances might be linked, Lindmeier’s reply stated: “Our investigations with the impacted countries are ongoing. To date we cannot confirm a link.”
Following the incidents of Indian syrups being flagged by other countries, the Indian Government has put in place a mechanism for all cough syrups meant for export to be tested before they are shipped out.
In a notification issued in May, the Indian Government said only those cough syrups that receive a ‘certificate of analysis’ from the country’s four central drug testing laboratories, two regional testing laboratories, or any of the NABL-accredited state testing laboratories, will be allowed for export.
The first incident came to light in October last year when the WHO raised its first medical product alert for contaminated syrups manufactured by Maiden Pharmaceuticals. The syrup was linked to the deaths of 70 children in the Gambia.
Uzbekistan reported the deaths of at least 18 children due to acute kidney injury linked to two syrups manufactured by Noida-based Marion Biotech.
In a similar instance in Indonesia, the death of around 200 children due to acute kidney injury were linked to eight contaminated syrups.
And, then an alert was raised by the Australian Drug Regulator for contaminated products found in Micronesia and Marshall islands. The medicines were manufactured by a Punjab-based QP Pharmachem, which has previously told The Indian Express that they never exported their syrup to these countries.
////
New estimates published this week in The Lancet indicate that more than 1·31 billion people could be living with diabetes by 2050 worldwide. That’s 1·31 billion people living with a disease that causes life-altering morbidity, high rates of mortality, and interacts with and exacerbates many other diseases. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(23)01296-5/fulltext
The increase in prevalence (up from 529 million in 2021) is expected to be driven by increases in type 2 diabetes, which in turn will be caused by a rise in the prevalence of obesity and by demographic shifts. In 2021, type 2 diabetes accounted for 90% of all diabetes prevalence. Most of this burden is attributable to social risk factors—such as high BMI, dietary risks, environmental and occupational risks, tobacco use, alcohol use, and low physical activity—that thrive on the obesogenic way our environments are designed and the inequitable way we organise our resources and societies.
Timed to coincide with the American Diabetes Association’s 83rd Scientific Session, The Lancet and The Lancet Diabetes and Endocrinology publish a Series on Global Inequity in Diabetes. Two papers—one global and one focused on the USA—together tell the unhappy and inequitable story of diabetes. By 2045, as many as three in four adults with diabetes will be living in low-income and middle-income countries. Currently, only 10% of people with diabetes living in these countries receive guideline-based diabetes care. Regardless of economic category, in every country, those who are discriminated against and marginalised suffer the most and worst consequences of diabetes. In the US, where the burden of type 2 diabetes in young people has nearly doubled in the past 20 years, the highest burden is seen among Black or Indigenous American populations.