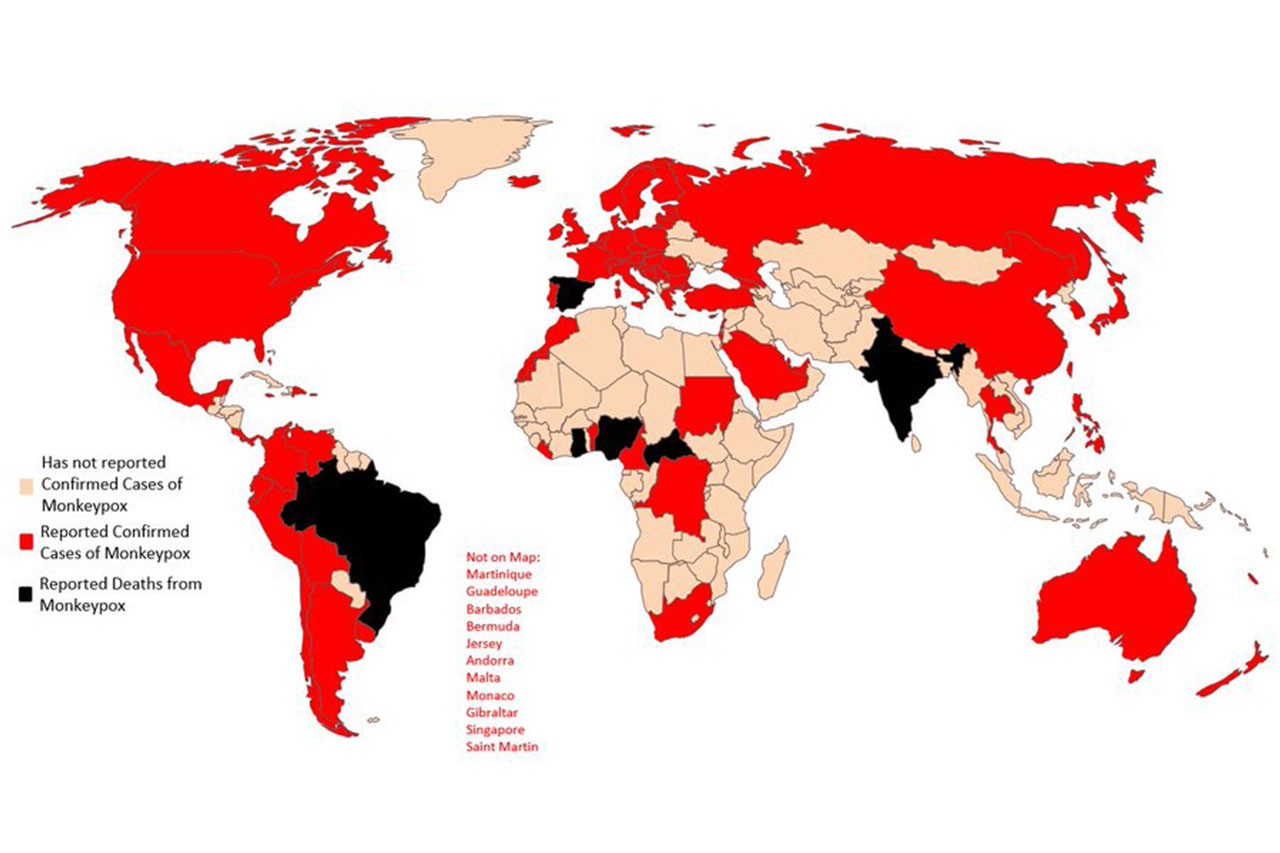
The Biden administration in the United States (US) has declared monkeypox a public health emergency.
There have been more than 7,000 cases detected in the US, but that is likely an undercount. The majority of cases are concentrated in the gay and queer community, primarily among men who have sex with men. But the Centers for Disease Control (CDC) and Prevention has also reported infections in a small number of cisgender women and at least two children.
Declaring a public health emergency in the US can trigger grant funding and open up more resources for various aspects of a federal response. It also allows the Department of Health and Human Services to enter into contracts for treatments and other necessary medical supplies and equipment, as well as support emergency hospital services, among other things. Public health emergencies last for 90 days, but can be extended.
CDC Director Rochelle Walensky said the declaration will provide resources and increase access to care. She also said it will expand the CDC’s ability to share data.
Epidemiologists and public health experts have warned that the US is running out of time to contain the outbreak.
Meanwhile, health officials in Britain said there were early signs that the spread of monkeypox was slowing. The country has some 2,900 confirmed cases of the virus.
///
US administration has decided to stretch out its limited supply of monkeypox vaccine by allowing a different method of injection that uses one-fifth as much per shot, according to people familiar with the discussions. www.nytimes.com/2022/08/08/us/politics/monkeypox-vaccine.html?
In order for the Food and Drug Administration (FDA) to authorize so-called intradermal injection, which would involve injecting one-fifth of the current dose into the skin instead of a full dose into underlying fat, the Department of Health and Human Services will need to issue a new emergency declaration allowing regulators to invoke the FDA’s emergency use powers. That declaration is expected as early as Tuesday afternoon.
The move would help alleviate a shortage of vaccine that has turned into a growing political and public health problem for the administration.
Even though it invested more than $1 billion in developing the two-dose vaccine known as Jynneos that works against both monkeypox and smallpox, the government has only 1.1 million shots on hand. It needs about three times as many doses to cover the 1.6 million to 1.7 million Americans who, according to the CDC, are at high risk of contracting monkeypox.
The vaccine is currently delivered in two 0.5-milliliter doses 28 days apart, with immune protection reaching its “maximum” 14 days after the second dose, according to the CDC.
The shot is recommended by the C.D.C. for people who have been exposed to monkeypox and those who might be likely to get it. Those in the latter category include people identified as a contact of someone with monkeypox, those who know a sexual partner from the last 14 days was diagnosed with the disease and those who have had “multiple” sexual partners in that time frame in an area with “known monkeypox.”
Federal health officials said last week that so far, they have distributed about 600,000 doses of the vaccine to state and local jurisdictions.
////
Japan’s health ministry on Tuesday approved KM Biologics freeze-dried smallpox vaccine LC16 KMB for use against monkeypox, after an experts’ panel recommended the move last week. www.medscape.com/viewarticle/978502?
KM Biologics, a unit of candy maker Meiji Holdings Co, has had “several inquiries from overseas” a company official told Reuters on Wednesday, declining to comment on any export plan for the shot.
Japan has had only two confirmed cases of monkeypox during the current global outbreak.
Based in the southern prefecture of Kumamoto, KM Biologics mainly produces vaccines for humans and veterinary use. It has an inactivated COVID-19 shot currently in development.
///
The European Medicines Agency (EMA) is recommending Novavax’s COVID-19 vaccine carry a warning of the possibility of two types of heart inflammation, an added burden for a shot that has so far failed to win wide uptake.( www.medscape.com/viewarticle/978527?)
The heart conditions – myocarditis and pericarditis – should be listed as new side-effects in the product information for the vaccine, Nuvaxovid, based on a small number of reported cases, the EMA said last Wednesday.
Novavax said no concerns about heart inflammations were raised during the clinical trials of Nuvaxovid and that more data would be gathered, adding that the most common cause of myocarditis is viral infections.
In June, the US FDA flagged a risk of heart inflammation from the Novavax vaccine.
Myocarditis and pericarditis were previously identified as rare side- effects, mostly seen in young men, from ground breaking messenger RNA (mRNA) vaccines made by Moderna and the Pfizer and BioNTech alliance, with the vast majority of those affected recovering fully.
The EMA said on Wednesday it had asked Novavax to provide additional data on the risk of these side-effects.
Last month, the EU agency identified severe allergic reactions as potential side-effects of the vaccine.
Novavax was hoping that people who have opted not to take Pfizer and Moderna’s vaccines would favour its shot because it relies on technology that has been used for decades to combat diseases including hepatitis B and influenza.
However, only around 250,000 doses of Nuvaxovid have been administered in Europe since its launch in December, according to the European Centers for Disease Prevention and Control.
///
North Korea says everyone who fell sick since the country confirmed its first Covid-19 infections has recovered. On Friday state media reported zero fever cases for a seventh straight day. North Korea refers to “fever” rather than “Covid” patients due to a lack of testing equipment. The country announced its first Covid outbreak in May and has reported fever infections and deaths since. But there is widespread doubt over the data, especially the number of deaths. “No new fever cases were reported during the past week and all those receiving treatment have recovered across the country,” the Korean Central News Agency (KCNA) reported on Friday.
https://www.bbc.co.uk/news/world-asia-62435809
///
New York state health officials have urged unvaccinated residents to get their polio shot “right away.”
One case of polio was confirmed last month in a county north of New York City, which the state’s health commissioner described as possibly “the tip of the iceberg.”
Wastewater samples taken in several locations north of New York City potentially signal community spread of the highly contagious disease.
/////
The world’s response to the HIV/AIDS pandemic is faltering badly in the face of declines in spending and the COVID-19 pandemic, according to an annual update from the Joint United Nations Programme on HIV/AIDS (UNAIDS) released last week. In a campaign announced in 2015 to “end AIDS as a public health threat” by 2030, UNAIDS set targets for 2025 that the new report finds are far from being met. Last year, 1.5 million people became infected with HIV, 1 million more than the 2025 target. Of the 38.4 million people living with the virus in 2021, 10 million are still not receiving lifesaving antiretroviral drugs, and last year saw the lowest number of new people starting treatment in a decade. Alarmingly, UNAIDS notes, 52% of infected children aren’t being treated.
www.science.org/content/article/news-glance-ai-protein-structures-racism-memory-rising-tiger-numbers
////
The British Heart Foundation will award £30 million ($36 million) over 5 years to an international team to develop genetic cures for some inherited heart diseases called cardiomyopathies. The group, dubbed CureHeart, won over three others shortlisted by the Big Beat Challenge, a competition launched in 2019 to fund transformative heart disease research. The team aims to use one-time injections of gene-editing tools to precisely correct or silence mutations that cause heart muscle cells to produce too little or a harmful form of a needed protein. These cardiomyopathies affect one in every 250 people, putting them at risk for heart attacks and heart failure; some will need a heart transplant. Within 5 years, CureHeart members in the United States, United Kingdom, and Singapore hope to develop one or more treatments to the point that companies will pick them up for clinical testing.
////
Lalita Panicker is Consulting Editor, Views and Editor, Insight, Hindustan Times, New Delhi

