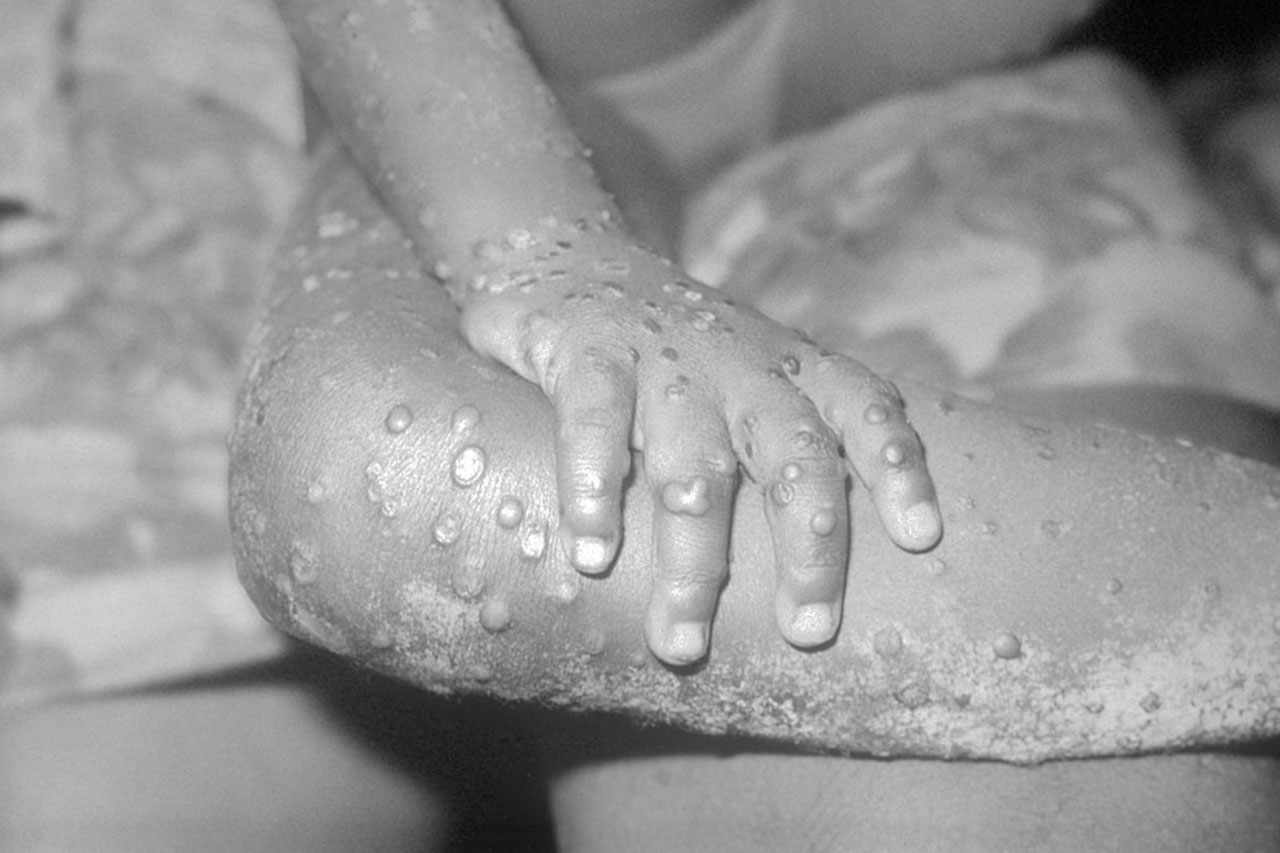
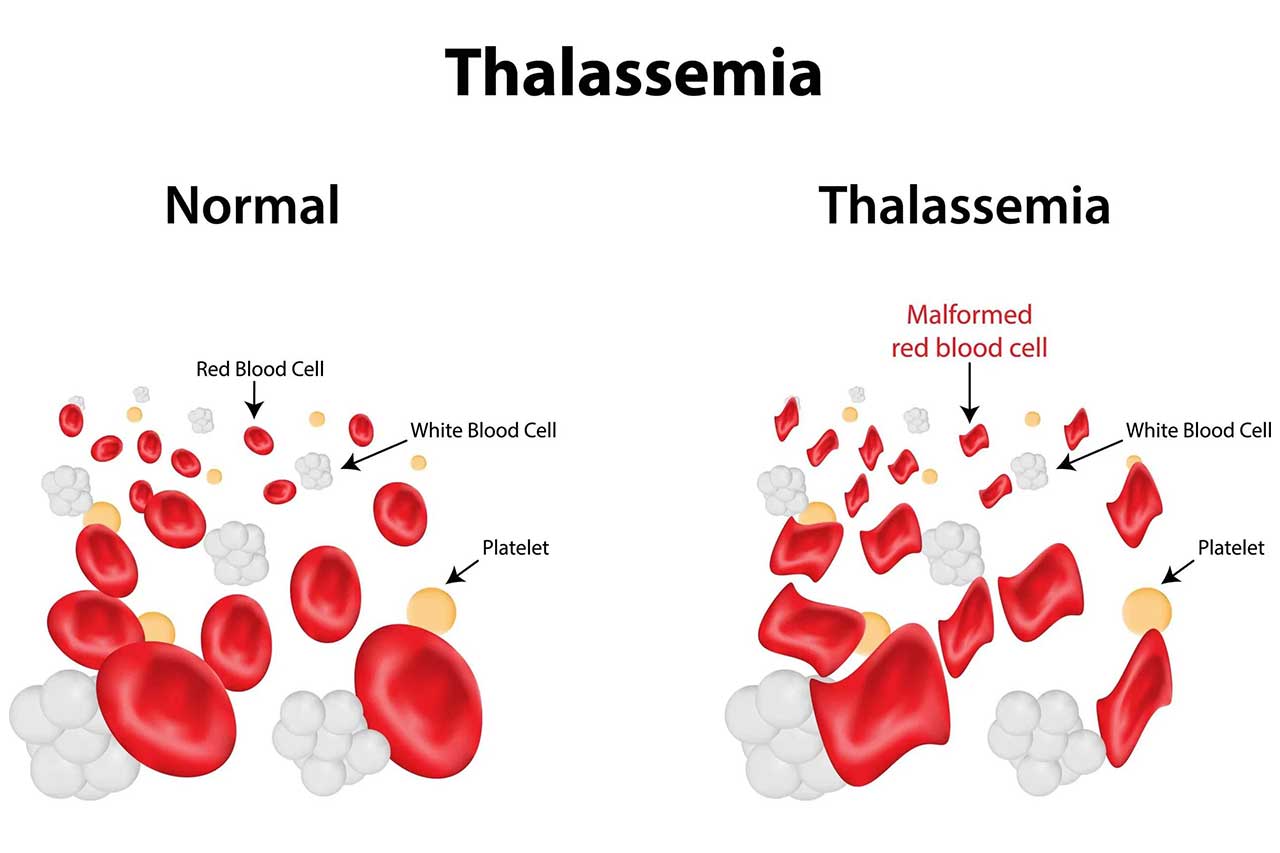
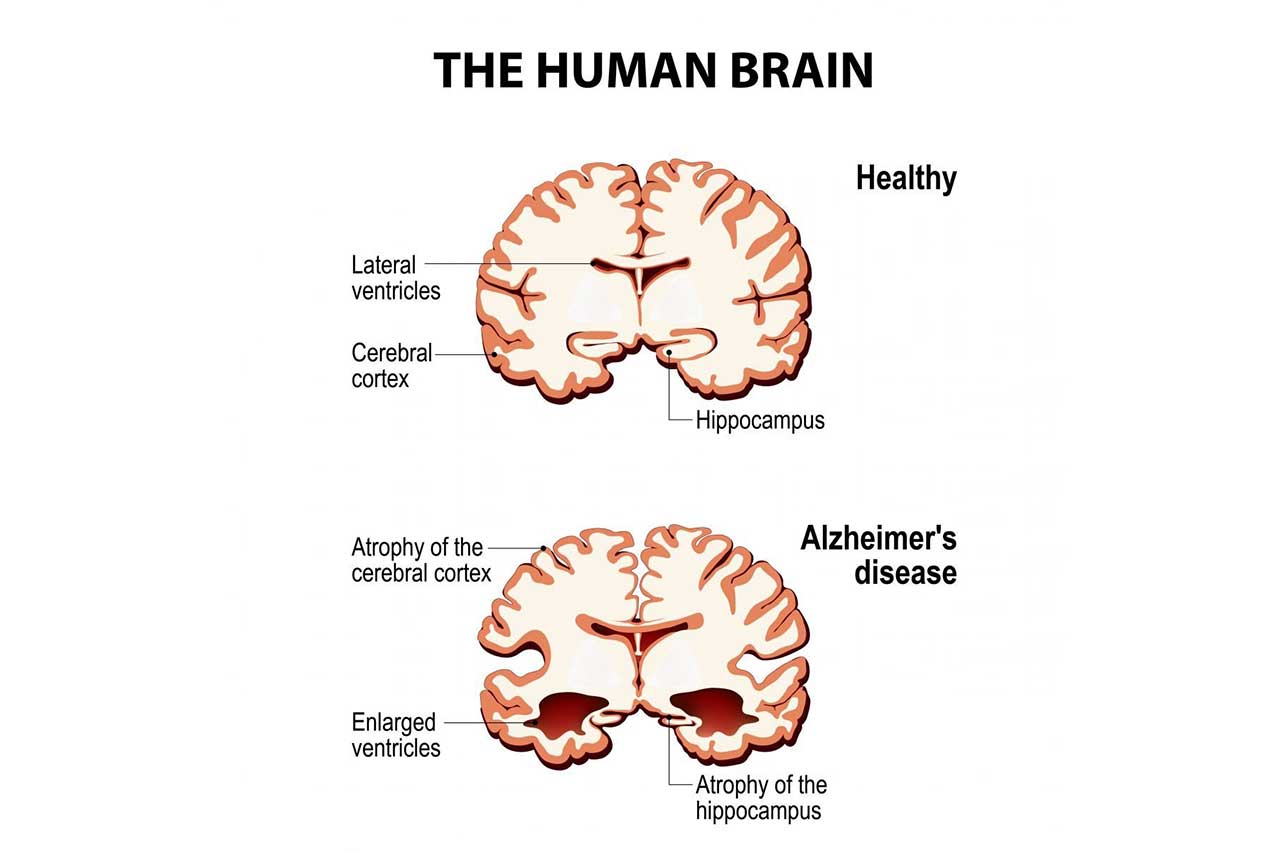
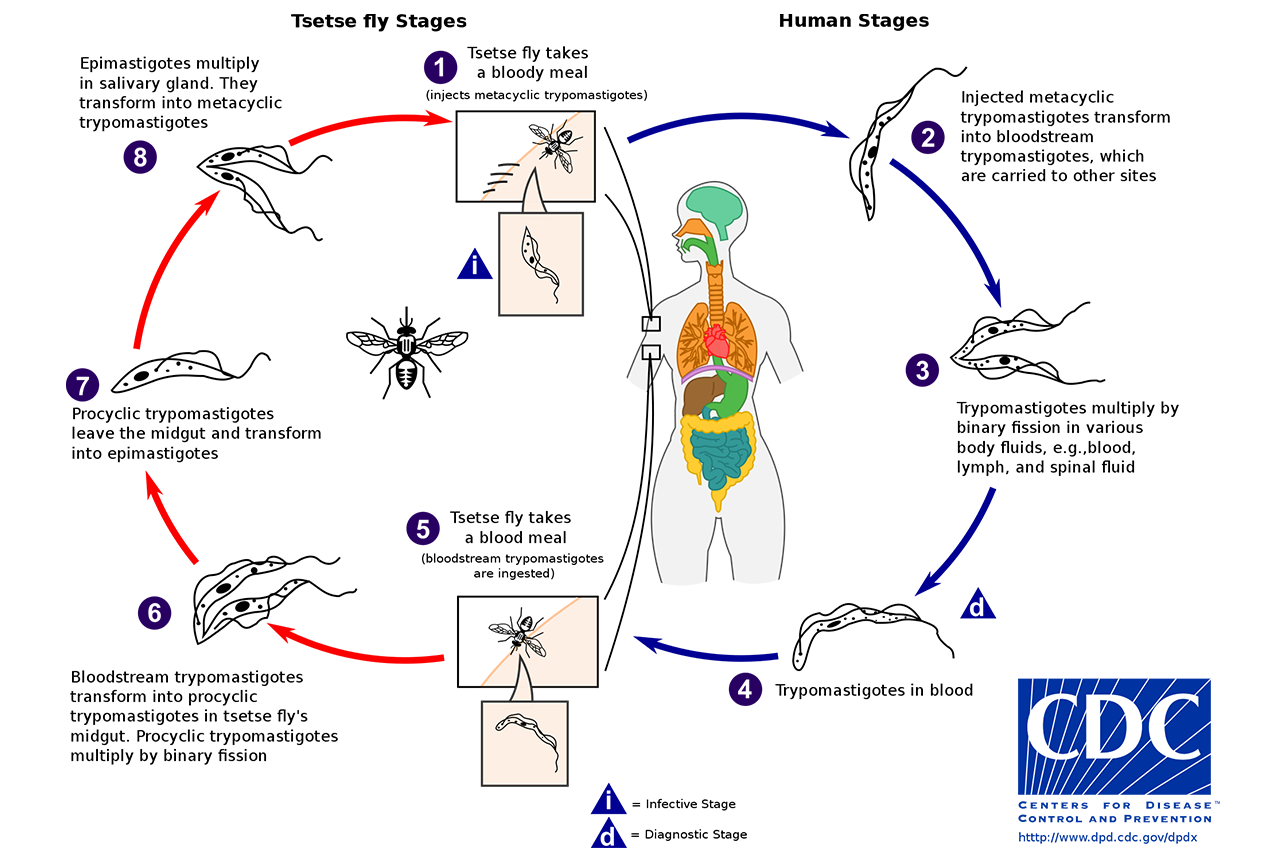
Article by Lalita Panicker, Consulting Editor, Views and Editor, Insight, Hindustan Times, New Delhi
At last, the world’s largest biomedical research agency has a permanent leader. The U.S. Senate recently voted 62-36 to confirm oncologist to direct the U.S. National...
Article by Lalita Panicker, Consulting Editor, Views and Editor, Insight, Hindustan Times, New Delhi
The inexpensive and widely used diabetes drug metformin decreased the risk of developing Long Covid in overweight and obese outpatients who took it while acutely ill...